
Contributions
Abstract: PB2012
Type: Publication Only
Background
Newly diagnosed classical Hodgkin lymphoma (cHL) is highly curable with multi-agent chemotherapy (CTx) +/- radiotherapy. However, standard-of-care treatment (Tx) for relapsed/refractory (R/R) cHL fails in ~50% of patients (pts). Recent clinical trials utilizing novel agents, including the PD-1 inhibitors nivolumab and pembrolizumab and the antibody–drug conjugate brentuximab vedotin (BV), have shown efficacy in R/R cHL. However, real-world clinical practice data describing effictiveness, safety, and pt experiences are limited. Better understanding of tumor biology and pt factors may impact Tx choices in cHL.
Aims
To describe current Tx patterns, evaluate clinical and pt-reported outcomes (PROs), and characterize tumor/pt molecular profiles in cHL pts.
Methods
This is an ongoing, observational, multicenter, prospective study (NCT02856646) involving ~80 US oncology practices with a target enrollment of 500 pts and a planned follow-up of ≤5 y. Eligible pts were ≥18 y with histologically confirmed cHL. At enrollment, pts were Tx naïve or within 2 wk of beginning any line of anticancer therapy. Informed consent was obtained for all pts. Index therapy was defined as therapy received at or within 2 wk of enrollment. Data were collected from existing medical records, by pt interaction using PRO questionnaires (FACT-Lym assessment), and by physician assessment. PRO data were collected every 3 mo during the 1st y of enrollment and every 6 mo thereafter. Blood and tissue samples were collected for biomarker analysis.
Results
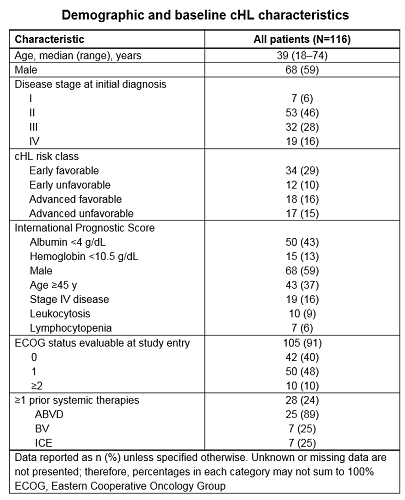
At data cut-off (Sept 2017), 116 pts were enrolled; 59% were male, median (range) age was 39 (18–74) y, and 46% had stage II disease at initial diagnosis (Table). At enrollment, 76% were Tx naïve and 24% had received prior therapy; index therapies were immunotherapy (3%) and CTx (94%). The most common index CTx were doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD; 73%); ifosfamide, carboplatin, and etoposide (ICE; 9%); and BV (9%). In total, 78%, 8%, and 3% of index therapies were induction, consolidation/intensification, and maintenance regimens, respectively. Complete response was achieved in 73% of pts who had completed index therapy. Of pts who discontinued index therapy, 33% and 17% discontinued due to relapse/progression and toxicity, respectively. Among pts with Tx-related adverse events (TRAEs), the most common were nausea (41%), fatigue (34%), neutropenia (31%), and constipation (25%). The most common concomitant medications received for TRAEs were ondansetron, filgrastim, and levofloxacin. At baseline, FACT-Lym v4 well-being assessments were available for 115 (99%) pts, (mean) scores were: physical (13.2), social (25.2), emotional (13.3), functional (19.8) and other concerns (25.9). Further PRO analyses will focus on differences between pt groups, stratifying by line of Tx and clinical characteristics over time. Biomarker analyses (9p24.1 amplification, PD-L1 and CD68 expression, Epstein-Barr virus status) for current pts are in process.
Conclusion
Initial data from the largest prospective observational study in cHL to date suggest that most pts receive multi-agent CTx as 1st-line Tx, consistent with the current Tx paradigm. Effectiveness, safety, and PRO analyses by pt subgroup and Tx line will continue as more pts are enrolled to identify any tumor biomarkers and pt factors which may correlate with clinical outcomes. Pt recruitment is ongoing.
Study support: BMS.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): chemotherapy, Clinical Trial, Hodgkin's Lymphoma, Immunotherapy
Abstract: PB2012
Type: Publication Only
Background
Newly diagnosed classical Hodgkin lymphoma (cHL) is highly curable with multi-agent chemotherapy (CTx) +/- radiotherapy. However, standard-of-care treatment (Tx) for relapsed/refractory (R/R) cHL fails in ~50% of patients (pts). Recent clinical trials utilizing novel agents, including the PD-1 inhibitors nivolumab and pembrolizumab and the antibody–drug conjugate brentuximab vedotin (BV), have shown efficacy in R/R cHL. However, real-world clinical practice data describing effictiveness, safety, and pt experiences are limited. Better understanding of tumor biology and pt factors may impact Tx choices in cHL.
Aims
To describe current Tx patterns, evaluate clinical and pt-reported outcomes (PROs), and characterize tumor/pt molecular profiles in cHL pts.
Methods
This is an ongoing, observational, multicenter, prospective study (NCT02856646) involving ~80 US oncology practices with a target enrollment of 500 pts and a planned follow-up of ≤5 y. Eligible pts were ≥18 y with histologically confirmed cHL. At enrollment, pts were Tx naïve or within 2 wk of beginning any line of anticancer therapy. Informed consent was obtained for all pts. Index therapy was defined as therapy received at or within 2 wk of enrollment. Data were collected from existing medical records, by pt interaction using PRO questionnaires (FACT-Lym assessment), and by physician assessment. PRO data were collected every 3 mo during the 1st y of enrollment and every 6 mo thereafter. Blood and tissue samples were collected for biomarker analysis.
Results
At data cut-off (Sept 2017), 116 pts were enrolled; 59% were male, median (range) age was 39 (18–74) y, and 46% had stage II disease at initial diagnosis (Table). At enrollment, 76% were Tx naïve and 24% had received prior therapy; index therapies were immunotherapy (3%) and CTx (94%). The most common index CTx were doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD; 73%); ifosfamide, carboplatin, and etoposide (ICE; 9%); and BV (9%). In total, 78%, 8%, and 3% of index therapies were induction, consolidation/intensification, and maintenance regimens, respectively. Complete response was achieved in 73% of pts who had completed index therapy. Of pts who discontinued index therapy, 33% and 17% discontinued due to relapse/progression and toxicity, respectively. Among pts with Tx-related adverse events (TRAEs), the most common were nausea (41%), fatigue (34%), neutropenia (31%), and constipation (25%). The most common concomitant medications received for TRAEs were ondansetron, filgrastim, and levofloxacin. At baseline, FACT-Lym v4 well-being assessments were available for 115 (99%) pts, (mean) scores were: physical (13.2), social (25.2), emotional (13.3), functional (19.8) and other concerns (25.9). Further PRO analyses will focus on differences between pt groups, stratifying by line of Tx and clinical characteristics over time. Biomarker analyses (9p24.1 amplification, PD-L1 and CD68 expression, Epstein-Barr virus status) for current pts are in process.

Conclusion
Initial data from the largest prospective observational study in cHL to date suggest that most pts receive multi-agent CTx as 1st-line Tx, consistent with the current Tx paradigm. Effectiveness, safety, and PRO analyses by pt subgroup and Tx line will continue as more pts are enrolled to identify any tumor biomarkers and pt factors which may correlate with clinical outcomes. Pt recruitment is ongoing.
Study support: BMS.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): chemotherapy, Clinical Trial, Hodgkin's Lymphoma, Immunotherapy


