
Contributions
Abstract: PB2292
Type: Publication Only
Background
Several studies have suggested the involvement of oxidative stress in aging and in many hematological disorders, including BCR-ABL1-negative myeloproliferative neoplasms. However, information is scarce about the oxidative status of patients with primary myelofibrosis (PMF) or myelofibrosis secondary to polycythemia vera (PV) or essential thrombocythemia (ET)1-9.
Aims
The major aim of this study is to evaluate the levels of reactive oxygen species and the total antioxidant capacity in patients with PMF and post-ET or post-PV myelofibrosis compared to healthy volunteers (control group). The minor aim is to observe whether the presence of the JAK2V617F mutation or of extramedullary hematopoiesis (EH) sites influenced the oxidative status of the study group.
Methods
We enrolled 10 patients with PMF or post-ET/post-PV myelofibrosis, hospitalized in the Clinic of Hematology, Filantropia City Hospital Craiova, and 20 healthy volunteers (control group). Informed consent was obtained from all subjects involved. Oxidative stress was evaluated using a CR3000 analyzer from a single drop of capillary blood. Reactive oxygen species were evaluated by FORT (Free Oxygen Radicals Testing) and the total antioxidant capacity by the FORD (Free Oxygen Radicals Defense) assays. The normal range for the FORT assay is < 2.3 mmol/L H2O2 and the normal range for the FORD assay is 1.07 – 1.53 mmol/L. The JAK2V617F mutation was detected by amplification refractory mutation system–polymerase chain reaction. EH sites were detected using computed tomography (CT), magnetic resonance imaging (MRI), cutaneous biopsy or scintigraphy. Statistical data analysis was performed using the student T-test and a p-value ≤ 0.05 was considered significant.
Results
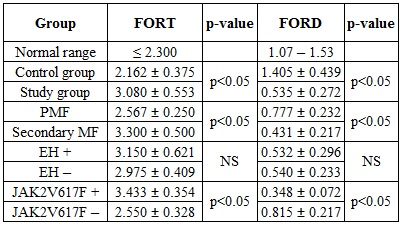
The study group involved 10 patients (median age = 63.0 ± 11.61 years, male-to-female ratio = 3:7) and 20 healthy controls (median age = 64.1 ± 2.26 years). Three patients had been diagnosed with PMF and seven patients developed post-PV or post-ET myelofibrosis. In PMF, scintigraphy revealed one lienal and one renal EH site and skin biopsy revealed one cutaneous EH site. In post-PV or post-ET myelofibrosis, CT detected one osseous EH site, MRI detected one lienal EH site and both CT and MRI confirmed a hepatic EH site. Data regarding the oxidative status are presented as mean value ± standard deviation in the attached table.
Conclusion
We found an unbalanced oxidative status in the study population compared to controls: levels of FORT were increased, whereas FORD levels were decreased. Post-PV or post-ET myelofibrosis cases and JAK2V617F-positive cases had higher FORT and lower FORD values than PMF cases and JAK2V617F-negative cases (p≤ 0.05). The presence of EH sites did not have an impact of oxidative stress status. We may hypothesize that oxidative stress is involved in the pathogenesis of PMF and post-PV or post-ET myelofibrosis and that the presence of the JAK2V617F mutation plays a role in the increase in oxidative stress levels.
References: 1. Gaman AM, et al. Aging Dis. 2016 May 27;7(3):307-17. doi: 10.14336/AD.2015.1022. 2. Gaman AM, et al. Oxid Med Cell Longev. 2014;2014:158135. doi: 10.1155/2014/158135. 3. Vener C, et al. Exp Hematol. 2010 Nov;38(11):1058-65. 4. Gaman MA, et al. Haematologica. 2017; 102(s2):835. abstract n. PB2105. 5. Gaman AM, et al. Haematologica. 2014; 99(s1):773. abstract n. PB2030. 6. Gaman MA, et al. Haematologica. 2017; 102(s2):840. abstract n. PB2119. 7. Gaman AM, et al. Haematologica. 2015; 100(s1):772. abstract n. PB1967.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Antioxidants, Extramedullary hematopoiesis, Myelofibrosis, Reactive oxygen species
Abstract: PB2292
Type: Publication Only
Background
Several studies have suggested the involvement of oxidative stress in aging and in many hematological disorders, including BCR-ABL1-negative myeloproliferative neoplasms. However, information is scarce about the oxidative status of patients with primary myelofibrosis (PMF) or myelofibrosis secondary to polycythemia vera (PV) or essential thrombocythemia (ET)1-9.
Aims
The major aim of this study is to evaluate the levels of reactive oxygen species and the total antioxidant capacity in patients with PMF and post-ET or post-PV myelofibrosis compared to healthy volunteers (control group). The minor aim is to observe whether the presence of the JAK2V617F mutation or of extramedullary hematopoiesis (EH) sites influenced the oxidative status of the study group.
Methods
We enrolled 10 patients with PMF or post-ET/post-PV myelofibrosis, hospitalized in the Clinic of Hematology, Filantropia City Hospital Craiova, and 20 healthy volunteers (control group). Informed consent was obtained from all subjects involved. Oxidative stress was evaluated using a CR3000 analyzer from a single drop of capillary blood. Reactive oxygen species were evaluated by FORT (Free Oxygen Radicals Testing) and the total antioxidant capacity by the FORD (Free Oxygen Radicals Defense) assays. The normal range for the FORT assay is < 2.3 mmol/L H2O2 and the normal range for the FORD assay is 1.07 – 1.53 mmol/L. The JAK2V617F mutation was detected by amplification refractory mutation system–polymerase chain reaction. EH sites were detected using computed tomography (CT), magnetic resonance imaging (MRI), cutaneous biopsy or scintigraphy. Statistical data analysis was performed using the student T-test and a p-value ≤ 0.05 was considered significant.
Results
The study group involved 10 patients (median age = 63.0 ± 11.61 years, male-to-female ratio = 3:7) and 20 healthy controls (median age = 64.1 ± 2.26 years). Three patients had been diagnosed with PMF and seven patients developed post-PV or post-ET myelofibrosis. In PMF, scintigraphy revealed one lienal and one renal EH site and skin biopsy revealed one cutaneous EH site. In post-PV or post-ET myelofibrosis, CT detected one osseous EH site, MRI detected one lienal EH site and both CT and MRI confirmed a hepatic EH site. Data regarding the oxidative status are presented as mean value ± standard deviation in the attached table.

Conclusion
We found an unbalanced oxidative status in the study population compared to controls: levels of FORT were increased, whereas FORD levels were decreased. Post-PV or post-ET myelofibrosis cases and JAK2V617F-positive cases had higher FORT and lower FORD values than PMF cases and JAK2V617F-negative cases (p≤ 0.05). The presence of EH sites did not have an impact of oxidative stress status. We may hypothesize that oxidative stress is involved in the pathogenesis of PMF and post-PV or post-ET myelofibrosis and that the presence of the JAK2V617F mutation plays a role in the increase in oxidative stress levels.
References: 1. Gaman AM, et al. Aging Dis. 2016 May 27;7(3):307-17. doi: 10.14336/AD.2015.1022. 2. Gaman AM, et al. Oxid Med Cell Longev. 2014;2014:158135. doi: 10.1155/2014/158135. 3. Vener C, et al. Exp Hematol. 2010 Nov;38(11):1058-65. 4. Gaman MA, et al. Haematologica. 2017; 102(s2):835. abstract n. PB2105. 5. Gaman AM, et al. Haematologica. 2014; 99(s1):773. abstract n. PB2030. 6. Gaman MA, et al. Haematologica. 2017; 102(s2):840. abstract n. PB2119. 7. Gaman AM, et al. Haematologica. 2015; 100(s1):772. abstract n. PB1967.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Antioxidants, Extramedullary hematopoiesis, Myelofibrosis, Reactive oxygen species


