
Contributions
Abstract: PB2318
Type: Publication Only
Background
Polycythemia vera (PV) is a Philadelphia chromosome-negative chronic myeloproliferative neoplasm characterized by mutation in Janus Kinase 2 (JAK2V617F), which is present in 95%–97% of PV patients. The most commonly used first-line cytoreductive agent is hydroxyurea (HU), although 1 in 4 patients become intolerant or resistant to HU.
Until 2016, interferon was the main second line treatment. However, the discovery of JAK2V617F mutation and the realization of the critical role that the JAK-STAT pathway has in the pathogenesis of the disease led to the development of the JAK1/2 inhibitor ruxolitinib. Use of ruxolitinib as a second line therapy has been shown to be efficacious in clinical trials; however, few real-life experiences have been reported to date.
Aims
The main aim of this study was a preliminary analysis of the effects of ruxolitinib treatment in HU-resistant or intolerant PV patients in a single center.
Methods
Five PV patients at our hematology service were hydroxyurea resistant or intolerant and were not currently included in clinical trials. Their date of diagnosis ranged from 1999 to 2014. Analytical parameters were extracted from routine analysis. The presence of the JAK2V617F mutation was detected by RT-PCR.
Results
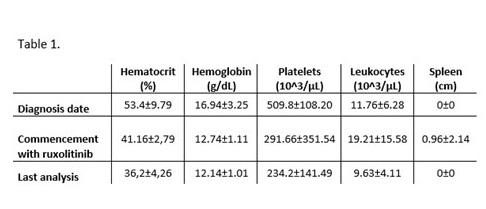
Of the 5 PV patients resistant or intolerant to hydroxyurea, 3 were women (60%). Three (60%) were aged 60 years and over at the date of diagnosis (mean age at diagnosis 60.4±1.67 years). Of the 5 PV patients, 3 (60%) were resistant to HU and 2 (40%) were intolerant. All patients presented the JAK2V617F mutation, with a mean allelic burden of 54.75 ± 31.76%. Maximum HU doses were 2 g/24 h (mean range 1.5 ± 0.71 g/24 h), and mean time with HU treatment was 6.4 ± 6.19 years. The mean hematological parameters of the PV patients at diagnosis, at time of commencement with ruxolitinib, and date of last analysis is shown in Table 1.
In general, adverse effects after commencement with ruxolitinib treatment reported were grade 1 or 2. Most common nonheamatological adverse events were asthenia, headache and pruritus. No patients has suffered any infections after of commencement with ruxolitinib treatment. With ruxolitinib, pruritus and asthenia symptoms improved for all patients, except in one patient who suffered a worsering of asthenia after commencement with ruxolitinib, but also an improvement of it after few months of treatment.. At the hematological level, there was a generalized recovery to normal levels with ruxolitinib treatment, especially platelet concentration. At the last analysis, no patients had progressed to secondary myelofibrosis and all patients were still alive.
Conclusion
Five HU-resistant or intolerant PV patients treated with ruxolitinib had a generalized improvement at the hematological level and an improvement of pruritus and asthenia symptoms. The outcomes of the outlined studies suggest that ruxolitinib is generally well tolerated as a second line treatment HU-resistant or intolerant PV patients. This preliminary study will be extended to include further patients to confirm these results.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Hydroxyurea, Polycythemia vera, Resistance, Ruxolitinib
Abstract: PB2318
Type: Publication Only
Background
Polycythemia vera (PV) is a Philadelphia chromosome-negative chronic myeloproliferative neoplasm characterized by mutation in Janus Kinase 2 (JAK2V617F), which is present in 95%–97% of PV patients. The most commonly used first-line cytoreductive agent is hydroxyurea (HU), although 1 in 4 patients become intolerant or resistant to HU.
Until 2016, interferon was the main second line treatment. However, the discovery of JAK2V617F mutation and the realization of the critical role that the JAK-STAT pathway has in the pathogenesis of the disease led to the development of the JAK1/2 inhibitor ruxolitinib. Use of ruxolitinib as a second line therapy has been shown to be efficacious in clinical trials; however, few real-life experiences have been reported to date.
Aims
The main aim of this study was a preliminary analysis of the effects of ruxolitinib treatment in HU-resistant or intolerant PV patients in a single center.
Methods
Five PV patients at our hematology service were hydroxyurea resistant or intolerant and were not currently included in clinical trials. Their date of diagnosis ranged from 1999 to 2014. Analytical parameters were extracted from routine analysis. The presence of the JAK2V617F mutation was detected by RT-PCR.
Results
Of the 5 PV patients resistant or intolerant to hydroxyurea, 3 were women (60%). Three (60%) were aged 60 years and over at the date of diagnosis (mean age at diagnosis 60.4±1.67 years). Of the 5 PV patients, 3 (60%) were resistant to HU and 2 (40%) were intolerant. All patients presented the JAK2V617F mutation, with a mean allelic burden of 54.75 ± 31.76%. Maximum HU doses were 2 g/24 h (mean range 1.5 ± 0.71 g/24 h), and mean time with HU treatment was 6.4 ± 6.19 years. The mean hematological parameters of the PV patients at diagnosis, at time of commencement with ruxolitinib, and date of last analysis is shown in Table 1.
In general, adverse effects after commencement with ruxolitinib treatment reported were grade 1 or 2. Most common nonheamatological adverse events were asthenia, headache and pruritus. No patients has suffered any infections after of commencement with ruxolitinib treatment. With ruxolitinib, pruritus and asthenia symptoms improved for all patients, except in one patient who suffered a worsering of asthenia after commencement with ruxolitinib, but also an improvement of it after few months of treatment.. At the hematological level, there was a generalized recovery to normal levels with ruxolitinib treatment, especially platelet concentration. At the last analysis, no patients had progressed to secondary myelofibrosis and all patients were still alive.

Conclusion
Five HU-resistant or intolerant PV patients treated with ruxolitinib had a generalized improvement at the hematological level and an improvement of pruritus and asthenia symptoms. The outcomes of the outlined studies suggest that ruxolitinib is generally well tolerated as a second line treatment HU-resistant or intolerant PV patients. This preliminary study will be extended to include further patients to confirm these results.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Hydroxyurea, Polycythemia vera, Resistance, Ruxolitinib


