
Contributions
Abstract: PB2284
Type: Publication Only
Background
There are well-known somatic driver mutations in genes JAK2, MPL, CALR associated with Ph-negative myeloproliferative neoplasms (MPN). Also somatic mutations in other genes (ASXL1, TET2, etc) are involved in the formation of the disease phenotype. Probably, there are some new mutations associated with MPN.
Aims
To carry out an advanced search for mutations in JAK2, MPL, CALR and ASXL1 using a next-generation sequencing–based method, with a MYELOID TUMOR SOLUTIONTM BY SOPHiA GENETICS panel for the patients with primary myelofibrosis (PMF).
Methods
4 patients with PMF were included in this study, their written informed consent for scientific evaluations were obtained. The diagnosis of all patients was confirmed by bone marrow trephine biopsies histological examination. Amplicon libraries were prepared by Myeloid Solution sequencing panel (SOPHiA GENETICS, Switzeland) and paired-end sequencing runs were performed on a MiSeq (Illumina, USA). MiSeq Reagent Kit v3 (600-cycle, Illumina) was used for direct sequencing. Dry bench was performed by SOPHiA DDM. In order to confirm the variants in ROI Sanger or pyrosequencing were performed.
Results
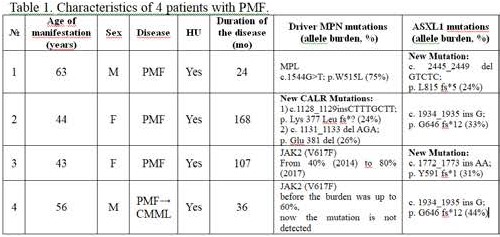
Characteristics of 4 patients with PMF are reported in Table 1. Patient №1 carries a known driver somatic mutation of MPL and new (here and below: not yet included in the COSMIC website) mutation in ASXL1. As a result of this mutation, there is a 5-bp deletion and a 2-bp frameshift that results in a mutant protein with a novel short C-terminus. Two new monoallelic driver mutations in oncogenic region of CALR gene were found in patient №2. The new mutant CALR fragment contains all the mutant amino acid sequence of the type I mutant L367fs*46 and nine altered and mainly positively charged amino acids LCLRRRRQR, therefore this new sequence has at least the same importance for oncogenicity as the type I CALR mutation. Also this patient carries ASXL1 mutation. Patient №2 suffers from PMF during 14 years without severe complications, which possibly means that mutation in ASXL1 simultaneously with new identified CALR mutation does not influence on survival rates. Patient №3 has V617F mutation and new mutation in ASXL1. There is a 2-bp insertion and a 2-bp frameshift that results in a mutant protein with a novel short C-terminus. The patient №4 had V617F mutation before October 2017. Currently, this mutation is not detected, which is probably due to the replacement of the V617F clone by another pathological clone. There are also somatic and germline mutations in genes KIT, SETBP1, TET, CBL, EZH2, SRSF2, which may be associated with chronic and acute leucosis (Greenman, 2007; Makishima, 2013; Hirsch, 2016) and ASXL1 mutation. Since August 2017 there is a worsening of the patient`s state and in the same time decreasing of allele burden of V617F mutation following complete extinction. There is a pathomorphologic data for transformaition of PMF into chronic meylomonocytic leucosis (CMML). Mentioned transformation is possibly intermediate step between PMF and acute leucosis partly due to epigenetic status caused by ASXL1 mutation.
Conclusion
Newly identified mutations in CALR and ASXL1 with high probability have diagnostic and prognostic meaning for patients with MPN.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): mutation analysis, Myelofibrosis, Myeloproliferative disorder, Somatic mutation
Abstract: PB2284
Type: Publication Only
Background
There are well-known somatic driver mutations in genes JAK2, MPL, CALR associated with Ph-negative myeloproliferative neoplasms (MPN). Also somatic mutations in other genes (ASXL1, TET2, etc) are involved in the formation of the disease phenotype. Probably, there are some new mutations associated with MPN.
Aims
To carry out an advanced search for mutations in JAK2, MPL, CALR and ASXL1 using a next-generation sequencing–based method, with a MYELOID TUMOR SOLUTIONTM BY SOPHiA GENETICS panel for the patients with primary myelofibrosis (PMF).
Methods
4 patients with PMF were included in this study, their written informed consent for scientific evaluations were obtained. The diagnosis of all patients was confirmed by bone marrow trephine biopsies histological examination. Amplicon libraries were prepared by Myeloid Solution sequencing panel (SOPHiA GENETICS, Switzeland) and paired-end sequencing runs were performed on a MiSeq (Illumina, USA). MiSeq Reagent Kit v3 (600-cycle, Illumina) was used for direct sequencing. Dry bench was performed by SOPHiA DDM. In order to confirm the variants in ROI Sanger or pyrosequencing were performed.
Results
Characteristics of 4 patients with PMF are reported in Table 1. Patient №1 carries a known driver somatic mutation of MPL and new (here and below: not yet included in the COSMIC website) mutation in ASXL1. As a result of this mutation, there is a 5-bp deletion and a 2-bp frameshift that results in a mutant protein with a novel short C-terminus. Two new monoallelic driver mutations in oncogenic region of CALR gene were found in patient №2. The new mutant CALR fragment contains all the mutant amino acid sequence of the type I mutant L367fs*46 and nine altered and mainly positively charged amino acids LCLRRRRQR, therefore this new sequence has at least the same importance for oncogenicity as the type I CALR mutation. Also this patient carries ASXL1 mutation. Patient №2 suffers from PMF during 14 years without severe complications, which possibly means that mutation in ASXL1 simultaneously with new identified CALR mutation does not influence on survival rates. Patient №3 has V617F mutation and new mutation in ASXL1. There is a 2-bp insertion and a 2-bp frameshift that results in a mutant protein with a novel short C-terminus. The patient №4 had V617F mutation before October 2017. Currently, this mutation is not detected, which is probably due to the replacement of the V617F clone by another pathological clone. There are also somatic and germline mutations in genes KIT, SETBP1, TET, CBL, EZH2, SRSF2, which may be associated with chronic and acute leucosis (Greenman, 2007; Makishima, 2013; Hirsch, 2016) and ASXL1 mutation. Since August 2017 there is a worsening of the patient`s state and in the same time decreasing of allele burden of V617F mutation following complete extinction. There is a pathomorphologic data for transformaition of PMF into chronic meylomonocytic leucosis (CMML). Mentioned transformation is possibly intermediate step between PMF and acute leucosis partly due to epigenetic status caused by ASXL1 mutation.

Conclusion
Newly identified mutations in CALR and ASXL1 with high probability have diagnostic and prognostic meaning for patients with MPN.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): mutation analysis, Myelofibrosis, Myeloproliferative disorder, Somatic mutation


