
Contributions
Abstract: PB2267
Type: Publication Only
Background
Since the discovery of CALR exon 9 mutations in 2013, the molecular diagnostic of ET and PMF has increased from approximately 60% (with diagnostic markers of JAK2 exon 14 and MPL exon 10 mutations) up to 90%. Generally, patients with mutant CALR exon 9 appear to have a prognostic advantage over those with wild type CALR. However, detection of ASXL1 mutations may enable more accurate assessment of risk stratification of PMF as these mutations are also frequently detected in PMF and appear to have a detrimental prognostic impact.
Aims
The aim of this study was to analyse CALR exon 9 mutation profiles of patients with ET and PMF and their association with ASXL1 exon 12 mutations in PMF.
Methods
For detection of mutations in CALR and ASXL1, CALR exon 9 and ASXL1 exon 12 respectively were PCR-amplified and direct sequencing of the amplicons was performed.
Results
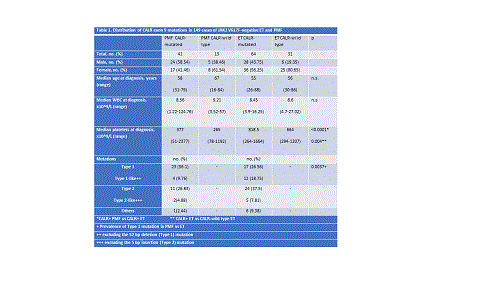
A cohort of 92 (56 females, 36 males) JAK2 V617F-negative ET and PMF patients from a single centre was first screened for CALR mutations. A total of 61% (20/33) of PMF and 47% (28/59) of ET patients were identified with CALR frameshift indel mutations. More than 10 different mutations including several other novel ones were observed whilst Type 1(n=21) and Type 2(n=12) were the common variants detected. There was also an apparent biased incidence of Type 1/Type 1-like mutations in PMF. Overall, CALR mutations occurred more frequently in males than in females (p=0.010).
For correlation studies of clinical data with mutation profiles, another 57 CALR-mutated patients (ET, n=36; PMF, n=21) were included. In 105 CALR-mutated patients (64 ET, 41 MF) studied, no significant difference was observed in the white blood cell counts or age between CALR-mutated and CALR-wild type patients within both ET and PMF cohorts. However, platelet count was significantly higher in CALR+ ET patients compared to the wild type cohort (p=0.004) and CALR+ PMF cohort (p<0.0001).
The higher frequency of Type 1 (CALR1) mutations in PMF than in ET (56% vs 27%, p=0.0037) were confirmed in this bigger cohort. Within PMF, platelet count was statistically higher in patients with Type 2-like (CALR2) mutations than those with CALR1.
All CALR+ PMF patients were also screened for ASXL1 exon 12 mutations. Of 41 patients analysed, 7 patients were positive with frameshift mutations and 3 with nonsense mutations. The most frequent mutation was c.1934dupG, which constituted 40% of mutations.
Although the study cohort size was small, the patients with ASXL1 mutations had significantly higher white blood cell count than those without (p=0.0035). Age, gender, platelet count and CALR mutation profile did not show any differences between different ASXL1 mutation status.
Conclusion
Like other studies which reported higher CALR1 prevalence in non-Asian populations, our cohort which comprised mostly Asians also showed similar profile. However, ASXL1 exon 12 mutations which were detected at a frequency of approximately 25% in CALR+ PMF in this study, did not appear to be associated with CALR1 or CALR2. It has been shown in large series that ASXL1 mutations compromised the survival of PMF patients while CALR mutations appearing to attenuate the effect. The interaction of CALR and ASXL1 mutations provides strong evidence for the prognostic relevance of performing CALR and ASXL1 mutation determination in all patients with PMF. This study can also be further expanded to a larger study population with survival analysis of patients based on mutational status and other risk factors.
Session topic: 15. Myeloproliferative neoplasms – Biology & Translational Research
Keyword(s): Essential Thrombocytemia, Mutation, Myelofibrosis
Abstract: PB2267
Type: Publication Only
Background
Since the discovery of CALR exon 9 mutations in 2013, the molecular diagnostic of ET and PMF has increased from approximately 60% (with diagnostic markers of JAK2 exon 14 and MPL exon 10 mutations) up to 90%. Generally, patients with mutant CALR exon 9 appear to have a prognostic advantage over those with wild type CALR. However, detection of ASXL1 mutations may enable more accurate assessment of risk stratification of PMF as these mutations are also frequently detected in PMF and appear to have a detrimental prognostic impact.
Aims
The aim of this study was to analyse CALR exon 9 mutation profiles of patients with ET and PMF and their association with ASXL1 exon 12 mutations in PMF.
Methods
For detection of mutations in CALR and ASXL1, CALR exon 9 and ASXL1 exon 12 respectively were PCR-amplified and direct sequencing of the amplicons was performed.
Results
A cohort of 92 (56 females, 36 males) JAK2 V617F-negative ET and PMF patients from a single centre was first screened for CALR mutations. A total of 61% (20/33) of PMF and 47% (28/59) of ET patients were identified with CALR frameshift indel mutations. More than 10 different mutations including several other novel ones were observed whilst Type 1(n=21) and Type 2(n=12) were the common variants detected. There was also an apparent biased incidence of Type 1/Type 1-like mutations in PMF. Overall, CALR mutations occurred more frequently in males than in females (p=0.010).
For correlation studies of clinical data with mutation profiles, another 57 CALR-mutated patients (ET, n=36; PMF, n=21) were included. In 105 CALR-mutated patients (64 ET, 41 MF) studied, no significant difference was observed in the white blood cell counts or age between CALR-mutated and CALR-wild type patients within both ET and PMF cohorts. However, platelet count was significantly higher in CALR+ ET patients compared to the wild type cohort (p=0.004) and CALR+ PMF cohort (p<0.0001).
The higher frequency of Type 1 (CALR1) mutations in PMF than in ET (56% vs 27%, p=0.0037) were confirmed in this bigger cohort. Within PMF, platelet count was statistically higher in patients with Type 2-like (CALR2) mutations than those with CALR1.
All CALR+ PMF patients were also screened for ASXL1 exon 12 mutations. Of 41 patients analysed, 7 patients were positive with frameshift mutations and 3 with nonsense mutations. The most frequent mutation was c.1934dupG, which constituted 40% of mutations.
Although the study cohort size was small, the patients with ASXL1 mutations had significantly higher white blood cell count than those without (p=0.0035). Age, gender, platelet count and CALR mutation profile did not show any differences between different ASXL1 mutation status.

Conclusion
Like other studies which reported higher CALR1 prevalence in non-Asian populations, our cohort which comprised mostly Asians also showed similar profile. However, ASXL1 exon 12 mutations which were detected at a frequency of approximately 25% in CALR+ PMF in this study, did not appear to be associated with CALR1 or CALR2. It has been shown in large series that ASXL1 mutations compromised the survival of PMF patients while CALR mutations appearing to attenuate the effect. The interaction of CALR and ASXL1 mutations provides strong evidence for the prognostic relevance of performing CALR and ASXL1 mutation determination in all patients with PMF. This study can also be further expanded to a larger study population with survival analysis of patients based on mutational status and other risk factors.
Session topic: 15. Myeloproliferative neoplasms – Biology & Translational Research
Keyword(s): Essential Thrombocytemia, Mutation, Myelofibrosis


