
Contributions
Abstract: PB2232
Type: Publication Only
Background
Lenalidomide in combination with dexamethasone is accepted for use in Scotland as 1st line therapy in transplant ineligible patients who are unsuitable for thalidomide treatment, 2nd line in patients who are unsuitable for thalidomide treatment and unrestricted use as 3rd line treatment.
Aims
This retrospective Real World Data (RWD) study examined the clinical efficacy and safety of lenalidomide in the treatment of multiple myeloma patients in NHS Greater Glasgow and Clyde (GG&C), with an approximate population of 1.2million.
Methods
All multiple myeloma patients treated in GG&C with lenalidomide from 01/01/2013 to 01/01/2016 were eligible for inclusion. Exclusion criteria were any patients with myeloma associated amyloidosis, those treated as part of a novel combination therapy or as part of maintenance therapy post-transplant. 131 patients met the criteria. Data were censored for 01/01/18. Patient characteristics including disease stage, ECOG status and renal function were determined. Clinical efficacy was evaluated using International Myeloma Working Group criteria for Time to Progression (TTP) and Progression Free Survival (PFS). Overall survival data were immature. Adverse effects and reasons for discontinuation were assessed.
Results
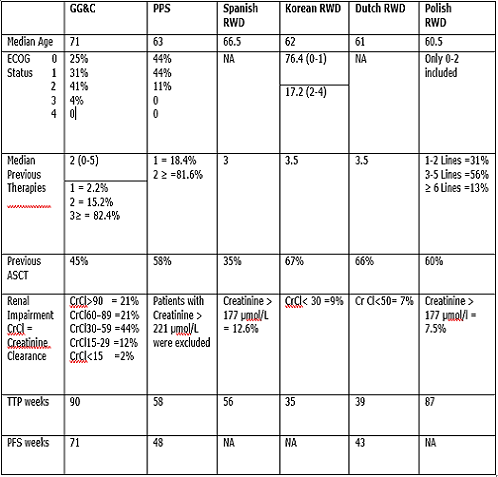
The overall median TTP was 90 weeks compared to the TTP of 58 weeks in the Pooled Pivotal Study1 (PPS) of the MM-009 and MM-010 trials. PFS was 71 weeks, compared to 48 weeks in the PPS. The improved PFS/TTP was despite patients being older and having poorer performance status and renal function to the PPS. The results compare favourably to Spanish2, Korean3, Dutch4 and Polish5 RWD studies (see table). TTP was found to be significantly higher than PFS. The TTP metric creates a less robust endpoint as it censors deaths that have not been positively attributed to disease progression. This challenges the applicability of TTP in myeloma patients, a primarily elderly group, who have higher mortality rates irrespective of their underlying malignancy. In total, 22% (n=29) of patients either discontinued lenalidomide due to adverse effects (n=18) or died (not attributable to progression) while on lenalidomide (n=11). Although generally higher, rates of discontinuation due to adverse effects and deaths were comparable to those in published literature.
Conclusion
This is the first study of lenalidomide use in unselected patients with a median age over 70 years and better reflects those treated in actual clinical practice. Despite the older age, poorer ECOG status and renal function, TTP/PFS were better than those seen in the PPS/RWD and confirms lenalidomide’s efficacy in an elderly and pre-treated patient group.
1. Dimopoulous, et al. Long-term follow-up on OS from the MM009 & MM010 phase III trials. 2009. Leuk. 23 2147-52
2. Allegre A, et al. Safety and efficacy of lenalidomide in Relapsed and Refractory Multiple Myeloma. 2012. Clinical MedicineInsights: Oncology. Vol 6: 1-10
3. Kim K, et al. Lenalidomide with dexamethasone treatment for Relapsed and Refractory Multiple Myeloma - experience from 110 patients. 2014. Annals of Haem. Vol 93:113-121
4. Kneppers E, et al. Analysis of efficacy and prognostic factors of lenalidomide treatment as part of a Dutch compassionate use. 2010. Clini Lymph, Myel and Leuk. Vol 10 (2): 138-143
5. Usnarska-Zubkiewicz. et al. Efficacy and safety of lenaliomide treatment in myeloma patients - The Polish Myeloma Group. 2016. Leukaemia Research. Vol 40: 90-99
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Elderly, Imids, Myeloma
Abstract: PB2232
Type: Publication Only
Background
Lenalidomide in combination with dexamethasone is accepted for use in Scotland as 1st line therapy in transplant ineligible patients who are unsuitable for thalidomide treatment, 2nd line in patients who are unsuitable for thalidomide treatment and unrestricted use as 3rd line treatment.
Aims
This retrospective Real World Data (RWD) study examined the clinical efficacy and safety of lenalidomide in the treatment of multiple myeloma patients in NHS Greater Glasgow and Clyde (GG&C), with an approximate population of 1.2million.
Methods
All multiple myeloma patients treated in GG&C with lenalidomide from 01/01/2013 to 01/01/2016 were eligible for inclusion. Exclusion criteria were any patients with myeloma associated amyloidosis, those treated as part of a novel combination therapy or as part of maintenance therapy post-transplant. 131 patients met the criteria. Data were censored for 01/01/18. Patient characteristics including disease stage, ECOG status and renal function were determined. Clinical efficacy was evaluated using International Myeloma Working Group criteria for Time to Progression (TTP) and Progression Free Survival (PFS). Overall survival data were immature. Adverse effects and reasons for discontinuation were assessed.
Results
The overall median TTP was 90 weeks compared to the TTP of 58 weeks in the Pooled Pivotal Study1 (PPS) of the MM-009 and MM-010 trials. PFS was 71 weeks, compared to 48 weeks in the PPS. The improved PFS/TTP was despite patients being older and having poorer performance status and renal function to the PPS. The results compare favourably to Spanish2, Korean3, Dutch4 and Polish5 RWD studies (see table). TTP was found to be significantly higher than PFS. The TTP metric creates a less robust endpoint as it censors deaths that have not been positively attributed to disease progression. This challenges the applicability of TTP in myeloma patients, a primarily elderly group, who have higher mortality rates irrespective of their underlying malignancy. In total, 22% (n=29) of patients either discontinued lenalidomide due to adverse effects (n=18) or died (not attributable to progression) while on lenalidomide (n=11). Although generally higher, rates of discontinuation due to adverse effects and deaths were comparable to those in published literature.

Conclusion
This is the first study of lenalidomide use in unselected patients with a median age over 70 years and better reflects those treated in actual clinical practice. Despite the older age, poorer ECOG status and renal function, TTP/PFS were better than those seen in the PPS/RWD and confirms lenalidomide’s efficacy in an elderly and pre-treated patient group.
1. Dimopoulous, et al. Long-term follow-up on OS from the MM009 & MM010 phase III trials. 2009. Leuk. 23 2147-52
2. Allegre A, et al. Safety and efficacy of lenalidomide in Relapsed and Refractory Multiple Myeloma. 2012. Clinical MedicineInsights: Oncology. Vol 6: 1-10
3. Kim K, et al. Lenalidomide with dexamethasone treatment for Relapsed and Refractory Multiple Myeloma - experience from 110 patients. 2014. Annals of Haem. Vol 93:113-121
4. Kneppers E, et al. Analysis of efficacy and prognostic factors of lenalidomide treatment as part of a Dutch compassionate use. 2010. Clini Lymph, Myel and Leuk. Vol 10 (2): 138-143
5. Usnarska-Zubkiewicz. et al. Efficacy and safety of lenaliomide treatment in myeloma patients - The Polish Myeloma Group. 2016. Leukaemia Research. Vol 40: 90-99
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Elderly, Imids, Myeloma


