
Contributions
Abstract: PB2165
Type: Publication Only
Background
Multiple myeloma (MM) is an incurable plasma cell malignancy. The primary goal of therapy is to obtain a deep and durable response to improve disease control and survival. In the post-transplant setting, large phase 3 trials suggest daily low-dose lenalidomide has a positive impact on progression free survival (PFS) and overall survival (OS).
Aims
We sought to evaluate the impact of lenalidomide maintenance in a real world setting in patients treated with bortezomib based induction chemotherapy and autologous stem cell transplant (ASCT) at our centre.
Methods
We evaluated all patients with MM from the Cross Cancer Institute who received frontline bortezomib-based induction chemotherapy and ASCT between December 2004 and January 2016 to ensure a minimum of 2 years follow-up. Patients were analyzed as receiving or not receiving maintenance based on intention-to-treat. Maintenance therapy involved lenalidomide monotherapy or in combination with bortezomib, an option at our center for patients with high risk cytogenetics. OS was measured from treatment initiation to death or last follow-up. PFS was measured from treatment initiation to relapse, death or last follow-up. Maximal response to treatment was assessed according to the International Myeloma Working Group criteria with an additional endpoint of near complete response (nCR) where CR was not confirmed by immunofixation or bone marrow biopsy.
Results
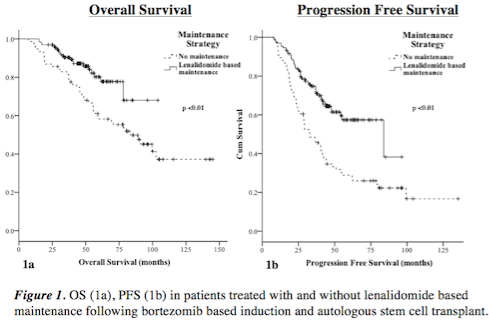
207 patients were included. 131 received lenalidomide based maintenance and 76 did not. Median ISS score was 2 in both groups (p=0.14). A mean of 28 cycles of lenalidomide (0.5 - 89) was given. 18% patients discontinued therapy prior to relapse. In the maintenance arm, 96% obtained a VGPR or greater compared to 76% in the no maintenance group (p <0.01). The median follow-up is 70 months in the non-maintenance cohort and 47 months in the maintenance cohort likely due to more recent adoption of lenalidomide use and therefore less time for follow-up in this cohort. The estimated 4-year OS was 87.1% in the maintenance group and 70.7% in the non-maintenance group (p <0.01, figure 1a). To date 105 patients have relapsed (47 in the maintenance and 58 in the non-maintenance cohort). The estimated 4-year PFS was 61.5% in the maintenance group and 34.6% in the non-maintenance group (p <0.01, figure 1b). The incidence of thromboembolism (TE) (3.1% vs 1.3% (p=0.43)) and second primary malignancies (SPMs) (2.3% vs 6.6% (p=0.12)) were low in the maintenance and non-maintenance groups respectively. 37.4% of patients in the maintenance group required dose adjustments. When first adopted, lenalidomide maintenance was given in a 28/28 day schedule and was pursued in 92 patients. Of these, 74.0% received all cycles at the intended dose. The current standard is a 21/28 day dosing schedule and was used in 38 patients. Of these, 77.6% received all cycles at the intended dose. In all, 18.3% of patients discontinued medication for reasons other than relapse.
Conclusion
Our data illustrates the positive impact of lenalidomide on depth of response, PFS and OS. Treatment was well tolerated with the majority of patients receiving the intended dose regimen. Few patients discontinued treatment prior to relapse. The rates of TEs and SPMs and consistent with previous reports. This data supports the ongoing use of lenalidomide maintenance in myeloma patients post-ASCT as a standard of care.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Imids, Maintenance, Myeloma, Survival
Abstract: PB2165
Type: Publication Only
Background
Multiple myeloma (MM) is an incurable plasma cell malignancy. The primary goal of therapy is to obtain a deep and durable response to improve disease control and survival. In the post-transplant setting, large phase 3 trials suggest daily low-dose lenalidomide has a positive impact on progression free survival (PFS) and overall survival (OS).
Aims
We sought to evaluate the impact of lenalidomide maintenance in a real world setting in patients treated with bortezomib based induction chemotherapy and autologous stem cell transplant (ASCT) at our centre.
Methods
We evaluated all patients with MM from the Cross Cancer Institute who received frontline bortezomib-based induction chemotherapy and ASCT between December 2004 and January 2016 to ensure a minimum of 2 years follow-up. Patients were analyzed as receiving or not receiving maintenance based on intention-to-treat. Maintenance therapy involved lenalidomide monotherapy or in combination with bortezomib, an option at our center for patients with high risk cytogenetics. OS was measured from treatment initiation to death or last follow-up. PFS was measured from treatment initiation to relapse, death or last follow-up. Maximal response to treatment was assessed according to the International Myeloma Working Group criteria with an additional endpoint of near complete response (nCR) where CR was not confirmed by immunofixation or bone marrow biopsy.
Results
207 patients were included. 131 received lenalidomide based maintenance and 76 did not. Median ISS score was 2 in both groups (p=0.14). A mean of 28 cycles of lenalidomide (0.5 - 89) was given. 18% patients discontinued therapy prior to relapse. In the maintenance arm, 96% obtained a VGPR or greater compared to 76% in the no maintenance group (p <0.01). The median follow-up is 70 months in the non-maintenance cohort and 47 months in the maintenance cohort likely due to more recent adoption of lenalidomide use and therefore less time for follow-up in this cohort. The estimated 4-year OS was 87.1% in the maintenance group and 70.7% in the non-maintenance group (p <0.01, figure 1a). To date 105 patients have relapsed (47 in the maintenance and 58 in the non-maintenance cohort). The estimated 4-year PFS was 61.5% in the maintenance group and 34.6% in the non-maintenance group (p <0.01, figure 1b). The incidence of thromboembolism (TE) (3.1% vs 1.3% (p=0.43)) and second primary malignancies (SPMs) (2.3% vs 6.6% (p=0.12)) were low in the maintenance and non-maintenance groups respectively. 37.4% of patients in the maintenance group required dose adjustments. When first adopted, lenalidomide maintenance was given in a 28/28 day schedule and was pursued in 92 patients. Of these, 74.0% received all cycles at the intended dose. The current standard is a 21/28 day dosing schedule and was used in 38 patients. Of these, 77.6% received all cycles at the intended dose. In all, 18.3% of patients discontinued medication for reasons other than relapse.

Conclusion
Our data illustrates the positive impact of lenalidomide on depth of response, PFS and OS. Treatment was well tolerated with the majority of patients receiving the intended dose regimen. Few patients discontinued treatment prior to relapse. The rates of TEs and SPMs and consistent with previous reports. This data supports the ongoing use of lenalidomide maintenance in myeloma patients post-ASCT as a standard of care.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Imids, Maintenance, Myeloma, Survival


