
Contributions
Abstract: PB2190
Type: Publication Only
Background
Multiple Myeloma (MM) is a hematologic neoplasm characterized by the proliferation of malignant clonal plasmacells in the bone marrow. The outcome of MM patients has significantly improved in the last years with the introduction of proteasome inhibitors (PI) and immunomodulatory (IMIDs) drugs as bortezomib and lenalidomide respectively. Besides, triplet regimens containing IMIDs with PI or monoclonal antibodies represent nowadays the backbone treatment for relapsed patients treatment. In detail, in the ASPIRE trial, the combination of second generation PI carfilzomib with lenalidomide and dexamethasone (KRD) led to a unprecedented results in term of high quality responses and progression free survival (PFS) with respect to lenalidomide-dexamethasone standard treatment. These results led to the approval in 2016 in Italy of KRD treatment for relapsed MM patients.
Aims
Here we report a multicentric real life experience of treatment with KRD in relapsed MM patients on behalf of the Gruppo Mieloma Triveneto.
Methods
A cohort of 80 patients affected by relapsed MM patients according to IMWG criteria was treated with KRD, in 10 hematological unit of Triveneto, Italy. Clinical characteristics, including response to treatment and toxicities, were collected.
Results
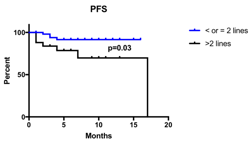
Median patient’s age was 62 years (42-76) with 32/80 patients (40%) with >65 years. Patients previously received a median of 2 lines of therapy (1-7), with 25/80 (31%) underwent to at least 3 regimens. Most patients (54/80, 68%) were treated due to clinical relapse, with 9/54 (17%) developing extramedullary disease, while only 26 patients (32%) received KRD due to biochemical relapse. With a median number of 5 cycles (1-17) of KRD received, the overall response rate (ORR) was 76% (61/80), including 40% of high quality response (21% Very Good Partial Response [VGPR] and 19% Complete Response [CR]). ORR and high quality response rate were higher although not significantly different among patients who previously received 1-2 lines of therapy towards patients who received >2 regimens (80% vs 65%, p=0.16 and 53% vs 35%, p=0.15). Eight patients (10%) during KRD treatment underwent to peripheral blood stem cells (PBSC) apheresis, 7 using high dose cyclophosphamide, and one using lenograstim with plerixafor. Almost all patients (7/8, 88%) completed PBSC collection, with a mean 7.39x106/Kg PBSC harvested. Besides, 7 patients already received stem cell transplantation, 5 using autologous PBSC and 2 using allogenic PBSC. Hematological toxicities were mild with neutropenia present in 27% of patients, thrombocytopenia in 26% and anemia in 11%; grade 3 toxicities were even lower (11%, 9% and 4% respectively). Non hematological toxicities were mostly infective, with 22 patients (28%) involved (with 8% of grade 3 events). Cardiovascular events including hypertension and heart failure were mild, with 8% of any grade events and only 5% grade 3 events. With a median follow up of 5 months, median PFS not reached; moreover, median PFS of patients who received at least 2 lines of therapy is already significantly higher than in patients who received >2 lines of therapy (17 months vs undefined, p=0.03).
Conclusion
In our experience, KRD treatment is feasible and effective in a non selected cohort of relapsed MM patients. The safety profile was acceptable, with main toxicities being represented by hematological and infective events, while cardiovascular side effects were fewer and less serious. Although our results need to be confirmed with a longer follow up, we point to the worst outcome in term of PFS of heavily treated patients.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Multiple Myeloma, Relapse, Therapy
Abstract: PB2190
Type: Publication Only
Background
Multiple Myeloma (MM) is a hematologic neoplasm characterized by the proliferation of malignant clonal plasmacells in the bone marrow. The outcome of MM patients has significantly improved in the last years with the introduction of proteasome inhibitors (PI) and immunomodulatory (IMIDs) drugs as bortezomib and lenalidomide respectively. Besides, triplet regimens containing IMIDs with PI or monoclonal antibodies represent nowadays the backbone treatment for relapsed patients treatment. In detail, in the ASPIRE trial, the combination of second generation PI carfilzomib with lenalidomide and dexamethasone (KRD) led to a unprecedented results in term of high quality responses and progression free survival (PFS) with respect to lenalidomide-dexamethasone standard treatment. These results led to the approval in 2016 in Italy of KRD treatment for relapsed MM patients.
Aims
Here we report a multicentric real life experience of treatment with KRD in relapsed MM patients on behalf of the Gruppo Mieloma Triveneto.
Methods
A cohort of 80 patients affected by relapsed MM patients according to IMWG criteria was treated with KRD, in 10 hematological unit of Triveneto, Italy. Clinical characteristics, including response to treatment and toxicities, were collected.
Results
Median patient’s age was 62 years (42-76) with 32/80 patients (40%) with >65 years. Patients previously received a median of 2 lines of therapy (1-7), with 25/80 (31%) underwent to at least 3 regimens. Most patients (54/80, 68%) were treated due to clinical relapse, with 9/54 (17%) developing extramedullary disease, while only 26 patients (32%) received KRD due to biochemical relapse. With a median number of 5 cycles (1-17) of KRD received, the overall response rate (ORR) was 76% (61/80), including 40% of high quality response (21% Very Good Partial Response [VGPR] and 19% Complete Response [CR]). ORR and high quality response rate were higher although not significantly different among patients who previously received 1-2 lines of therapy towards patients who received >2 regimens (80% vs 65%, p=0.16 and 53% vs 35%, p=0.15). Eight patients (10%) during KRD treatment underwent to peripheral blood stem cells (PBSC) apheresis, 7 using high dose cyclophosphamide, and one using lenograstim with plerixafor. Almost all patients (7/8, 88%) completed PBSC collection, with a mean 7.39x106/Kg PBSC harvested. Besides, 7 patients already received stem cell transplantation, 5 using autologous PBSC and 2 using allogenic PBSC. Hematological toxicities were mild with neutropenia present in 27% of patients, thrombocytopenia in 26% and anemia in 11%; grade 3 toxicities were even lower (11%, 9% and 4% respectively). Non hematological toxicities were mostly infective, with 22 patients (28%) involved (with 8% of grade 3 events). Cardiovascular events including hypertension and heart failure were mild, with 8% of any grade events and only 5% grade 3 events. With a median follow up of 5 months, median PFS not reached; moreover, median PFS of patients who received at least 2 lines of therapy is already significantly higher than in patients who received >2 lines of therapy (17 months vs undefined, p=0.03).

Conclusion
In our experience, KRD treatment is feasible and effective in a non selected cohort of relapsed MM patients. The safety profile was acceptable, with main toxicities being represented by hematological and infective events, while cardiovascular side effects were fewer and less serious. Although our results need to be confirmed with a longer follow up, we point to the worst outcome in term of PFS of heavily treated patients.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Multiple Myeloma, Relapse, Therapy


