
Contributions
Abstract: PB2237
Type: Publication Only
Background
Phase IIIB UPFRONT trial was designed to compare three frontline bortezomib-based regimens in transplantation ineligible patients with Myeloma Multiple (MM) (Niesvizky R, et al. JCO 2015;33:3921-3929. Median progression free survival (PFS) with VD, VTD and VMP was 14.7, 15.4 and 17.3 months, respectively; median overall survival was 49.8, 51.5 and 53.1 months. Nowadays, lenalidomide continuous frontline therapy in elderly MM patients has showed better results in PSF and OS in this group of patients.
Aims
The aim of our review was to evaluate the efficacy and clinical outcome of initial bortezomib-based therapies and the maintenance treatment with thalidomide in elderly MM patients.
Methods
we report a total number of 30 elderly MM patients (age median 80 years old, range 66-89; 19 females) since January 2008 to December 2017 . 18 patients were treated in frontline with Bortezomib and Dexamethasone (VD), 8 patients with Bortezomib. Melphalan and prednisone (VMP) and 5 patients with Bortezomib. Cyclophosphamide and dexamethasone (VCD). The patients after completed the planned cycles of Bortezomib (median 7 cycles, range 3-14), received maintenance therapy with oral thalidomide (50mg/d) until disease progression or toxicity. Response rate, progression-free survival (PFS) and overall survival (OS) were the outcome measures.
Results
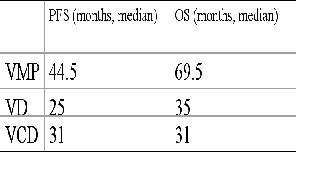
Nowadays, 6 patients are ongoing with thalidomide therapy. 11 (36.6%) patients had to stopped the thalidomide due to progression of disease. Only one patient (3,3%) had discontinued thalidomide therapy by tolerability. In overall group, we reported a PFS of 33,5 median months, and 35 median months of OS. In the table 1, we described the results of PFS and OS, in the patient’s different subgroup.
Conclusion
1) Thalidomide maintenance offer an advantage in PFS and OS in all bortezomib containing regimens (VTD; VD; VCD) in transplantation ineligible patients with MM; 2) Thalidomide maintenance was feasible without producing cumulative toxicity. 3) The continuous treatment with thalidomide (low dosage, 50 mg/d) is efficacious, tolerable and low cost, and it should be taken into consideration.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): bortezomib, Maintenance, Myeloma, Thalidomide
Abstract: PB2237
Type: Publication Only
Background
Phase IIIB UPFRONT trial was designed to compare three frontline bortezomib-based regimens in transplantation ineligible patients with Myeloma Multiple (MM) (Niesvizky R, et al. JCO 2015;33:3921-3929. Median progression free survival (PFS) with VD, VTD and VMP was 14.7, 15.4 and 17.3 months, respectively; median overall survival was 49.8, 51.5 and 53.1 months. Nowadays, lenalidomide continuous frontline therapy in elderly MM patients has showed better results in PSF and OS in this group of patients.
Aims
The aim of our review was to evaluate the efficacy and clinical outcome of initial bortezomib-based therapies and the maintenance treatment with thalidomide in elderly MM patients.
Methods
we report a total number of 30 elderly MM patients (age median 80 years old, range 66-89; 19 females) since January 2008 to December 2017 . 18 patients were treated in frontline with Bortezomib and Dexamethasone (VD), 8 patients with Bortezomib. Melphalan and prednisone (VMP) and 5 patients with Bortezomib. Cyclophosphamide and dexamethasone (VCD). The patients after completed the planned cycles of Bortezomib (median 7 cycles, range 3-14), received maintenance therapy with oral thalidomide (50mg/d) until disease progression or toxicity. Response rate, progression-free survival (PFS) and overall survival (OS) were the outcome measures.
Results
Nowadays, 6 patients are ongoing with thalidomide therapy. 11 (36.6%) patients had to stopped the thalidomide due to progression of disease. Only one patient (3,3%) had discontinued thalidomide therapy by tolerability. In overall group, we reported a PFS of 33,5 median months, and 35 median months of OS. In the table 1, we described the results of PFS and OS, in the patient’s different subgroup.

Conclusion
1) Thalidomide maintenance offer an advantage in PFS and OS in all bortezomib containing regimens (VTD; VD; VCD) in transplantation ineligible patients with MM; 2) Thalidomide maintenance was feasible without producing cumulative toxicity. 3) The continuous treatment with thalidomide (low dosage, 50 mg/d) is efficacious, tolerable and low cost, and it should be taken into consideration.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): bortezomib, Maintenance, Myeloma, Thalidomide


