
Contributions
Abstract: PB2211
Type: Publication Only
Background
Lenalidomide (LEN) is an important backbone of regimens in the multiple myeloma (MM) treatment arsenal. Non-adherence (NA) to anticancer therapy occurs in approximately 30% of cancer patients and is generally associated with adverse outcomes. A recent study used pharmacy refill data to show LEN implementation NA in 14.5% of MM patients (defined as medication possession ratio <80%), but ON-OFF cycling with LEN can affect the reliability of such data. Electronic methods of measuring adherence provide richer sampling and better reliability. There is no prior prospective longitudinal data on LEN adherence, and specifically no reports on electronically measured (EM) LEN implementation adherence.
Aims
(1) To determine the period prevalence of EM implementation adherence to LEN; (2) To delineate patterns of ON-OFF cycling
Methods
Methods: We report on an ad-hoc interim analysis of a prospective observational pilot cohort study of LEN-naïve adult patients receiving LEN-based regimens for treatment of MM. Patients completing follow-up by Feb 1, 2018 were included in this analysis. Convenience sampling was used to enroll 37 patients cared for in the hematology outpatient clinic (all from 1 tertiary center), 34 of whom were included in this analysis (3 excluded due to technical defects or inability to use the EM device). Sample characteristics were as follows: median age, 64.5 years [interquartile range (IQR) 58-74]; 35% female; median no. of medications, 5 [IQR 3-6]; median ISS, 2 [IQR 2-3]; median prior disease duration, 22.5 months [IQR 6.5-56]; LEN regimen: 71% LEN-dexamethasone; line of treatment: 1st = 6%, 2nd = 73%, 3rd = 21%.
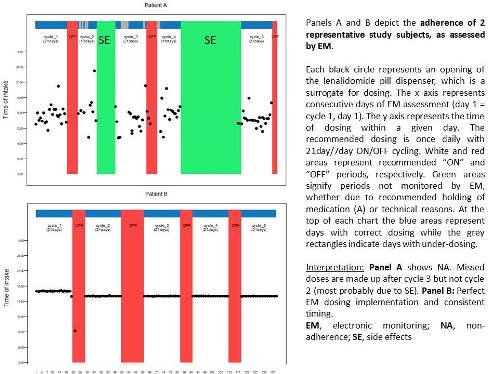
Implementation adherence to once daily LEN in 21day/7day (ON/OFF) cycles was monitored using EM (MEMS®, AARDEX) from study index (i.e. LEN cycle1, day1) until the end of cycle 5. This provided data on daily dosing (Figure 1). Adherence was expressed as the percentage of days with LEN taken as prescribed during ON cycling, independent of dosing timing. Descriptive statistics were used to show the median [IQR] for continuous variables and percentages for categorical data.
Results
Median LEN implementation adherence was 98.9% (IQR 93.1-100%; min 51.7%), while the mean was 93.8% (i.e. % of days with correct dosing during ON cycling). Representative cases of suboptimal and perfect adherers are shown in Figure 1. The median duration of follow-up was 132 days (IQR 76-138), while the median no. of cycles was 4.5 (IQR 2-5). The reasons for completing less than 5 LEN cycles with EM (n=17) were as follows: treatment change (n=7) mostly due to side effects (n=4); withdrawal of consent (n=5); death (n=1); others (n=4). 41% of patients had no adjustment in ON cycle length, while 53% had ≥1 shortened cycles. 80% (24/30) of evaluable patients achieved partial response or more at 6 months.
Conclusion
LEN implementation adherence was remarkably high yet showed large variability in this pilot study. Most patients had adjustments in cycle length, emphasizing the limitations of adherence analyses assuming fixed 21/7day cycling and the importance of rich sampling (e.g. EM). Importantly, a quarter of patients had correct dosing on less than 93% of days, which may be suboptimal. These patients may be candidates for adherence enhancing interventions. Thus, future research should focus on strategies for identifying this small subgroup of non-adherers.
IA and HM contributed equally; AL and AN contributed equally.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Immunomodulatory thalidomide analog, Myeloma, Treatment
Abstract: PB2211
Type: Publication Only
Background
Lenalidomide (LEN) is an important backbone of regimens in the multiple myeloma (MM) treatment arsenal. Non-adherence (NA) to anticancer therapy occurs in approximately 30% of cancer patients and is generally associated with adverse outcomes. A recent study used pharmacy refill data to show LEN implementation NA in 14.5% of MM patients (defined as medication possession ratio <80%), but ON-OFF cycling with LEN can affect the reliability of such data. Electronic methods of measuring adherence provide richer sampling and better reliability. There is no prior prospective longitudinal data on LEN adherence, and specifically no reports on electronically measured (EM) LEN implementation adherence.
Aims
(1) To determine the period prevalence of EM implementation adherence to LEN; (2) To delineate patterns of ON-OFF cycling
Methods
Methods: We report on an ad-hoc interim analysis of a prospective observational pilot cohort study of LEN-naïve adult patients receiving LEN-based regimens for treatment of MM. Patients completing follow-up by Feb 1, 2018 were included in this analysis. Convenience sampling was used to enroll 37 patients cared for in the hematology outpatient clinic (all from 1 tertiary center), 34 of whom were included in this analysis (3 excluded due to technical defects or inability to use the EM device). Sample characteristics were as follows: median age, 64.5 years [interquartile range (IQR) 58-74]; 35% female; median no. of medications, 5 [IQR 3-6]; median ISS, 2 [IQR 2-3]; median prior disease duration, 22.5 months [IQR 6.5-56]; LEN regimen: 71% LEN-dexamethasone; line of treatment: 1st = 6%, 2nd = 73%, 3rd = 21%.
Implementation adherence to once daily LEN in 21day/7day (ON/OFF) cycles was monitored using EM (MEMS®, AARDEX) from study index (i.e. LEN cycle1, day1) until the end of cycle 5. This provided data on daily dosing (Figure 1). Adherence was expressed as the percentage of days with LEN taken as prescribed during ON cycling, independent of dosing timing. Descriptive statistics were used to show the median [IQR] for continuous variables and percentages for categorical data.
Results
Median LEN implementation adherence was 98.9% (IQR 93.1-100%; min 51.7%), while the mean was 93.8% (i.e. % of days with correct dosing during ON cycling). Representative cases of suboptimal and perfect adherers are shown in Figure 1. The median duration of follow-up was 132 days (IQR 76-138), while the median no. of cycles was 4.5 (IQR 2-5). The reasons for completing less than 5 LEN cycles with EM (n=17) were as follows: treatment change (n=7) mostly due to side effects (n=4); withdrawal of consent (n=5); death (n=1); others (n=4). 41% of patients had no adjustment in ON cycle length, while 53% had ≥1 shortened cycles. 80% (24/30) of evaluable patients achieved partial response or more at 6 months.

Conclusion
LEN implementation adherence was remarkably high yet showed large variability in this pilot study. Most patients had adjustments in cycle length, emphasizing the limitations of adherence analyses assuming fixed 21/7day cycling and the importance of rich sampling (e.g. EM). Importantly, a quarter of patients had correct dosing on less than 93% of days, which may be suboptimal. These patients may be candidates for adherence enhancing interventions. Thus, future research should focus on strategies for identifying this small subgroup of non-adherers.
IA and HM contributed equally; AL and AN contributed equally.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Immunomodulatory thalidomide analog, Myeloma, Treatment


