
Contributions
Abstract: PB2188
Type: Publication Only
Background
Carfilzomib, Lenalidomide and Dexamethazone (KRd) is currently utilized in relapsed/refractory multiple myeloma (RRMM) patients (pts) that have experienced almost one line of treatment. Improved progression free survival and overall survival was detected in this setting of pts treated with this combination in clinical trials. Although the onset of new cardiovascular events (CV) or worsening of pre-existing CV events have been described in less than 10% of pts, the regimen has an accettable safety and tolerability. Since its approval in Italy, data on real life KRd use are still lacking.
Aims
We conducted an analysis of RRMM pts treated with KRd out of clinical trials to provide further insights on efficacy, tolerability and safety.
Methods
From November 2016 up to now 35 RRMM pts were exposed to KRd. Median number of prior therapies was 2 (range 1-6); 16 (45%) was classified as high risk pts (r-ISS) and 17 (48%) were previously autotransplanted.
Results
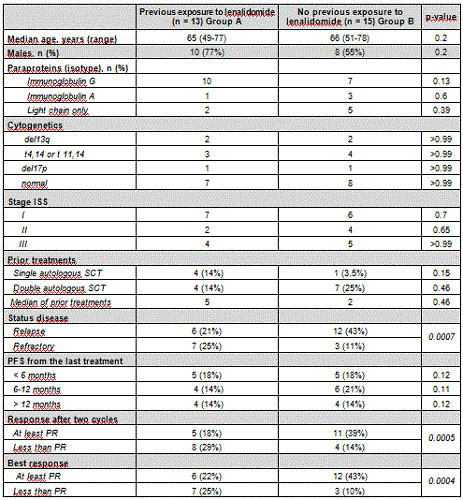
We report results of the first 12-months safety interim analysis on KRD treatment of RRMM. Data cut-off was January 2018. 35 pts with a median age of 65 years (range 50-79) were evaluated. All the pts received almost 2 cycles, 1 pts 16 cycles, 2 pts 15 cycles, 3 pts 14 cycles, 2 pts 13 cycles, 2 pts 12 cycles, 3 pts 11 cycles, 2 pts 10 and 9 cycles, 5 pts 8 cycles, 2 pts 7, 6, 5 and 4, 2 pts only 3 cycles, 3 pts only 2 cycles, At least 5 KRD treatment cycles were given to 28 (80%) pts. All pts had a response to treatment at the first/second cycle. At the end of the second cycle 26 (74%) pts obtained a response. Overall response rate with KRD was nearly 80% among the 28 pts that have completed 5 cycles.The majority of the pts(n=26, 96%) responded after at least 1 cycle of chemotherapy. Partial response were observed in 6 pts(21%). Rate of very good PR was 32% (9 pts); 3 pts(10%) achieved complete response (CR), 1 patient minimal response (MR), 6 pts obtained a stable disease, 6 pts obtained a stable response and then progressed. To identify pts that could obtain the most advantage by KRd treatment, we distinguished pts that have completed almost three cycles in two groups, based on previous exposure or not to lenalidomide (Table1). The 13 pts previously treated with lenalidomide (group A), compared to 15 pts not exposed to lenalidomide (group B), showed a worst profile: in the group A there was a higher % of refractory pts(25% vs 11%, p=0.0007) who received more prior treatments, despite the duration of the last treatment was similar in both groups. The regimen was well tolerated. with grade 3-4 haematological and non-haematological AEs on 10 (35%) and 15 (53%) pts respectively. Grade 3-4 non- haematological AEs occurred in 53% of patients, the most common being pneumonia/fever (3/2, 10/7%), hypertension (8, 28%) hiperglicemia (1, 3%), and cardiac failure (2, 7%). Cardiac AEs were 35% (10 pts). Pts with ≥1 CV risk factor at enrolment had a an increased risk of developing a CV AE during treatment as compared to pts with no CV risk factors.1 pt died of infection (not treatment-related). In pts who developed serious AE, KRD dose reduction (2, 7%) and discontinuation (3, 10%) were applied. Onset of CV events significantly increased the rate of dose reductions and treatment discontinuation. CV AEs may significantly impact treatment compliance and survival.
Conclusion
Rate of VGPR obtained with KRd combination was high with an overall response of about 80%. Safety profile was acceptable with a 35% of CV events. Additional analyses are needed to evaluate the impact of these patterns on efficacy outcomes.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Myeloma, Relapse
Abstract: PB2188
Type: Publication Only
Background
Carfilzomib, Lenalidomide and Dexamethazone (KRd) is currently utilized in relapsed/refractory multiple myeloma (RRMM) patients (pts) that have experienced almost one line of treatment. Improved progression free survival and overall survival was detected in this setting of pts treated with this combination in clinical trials. Although the onset of new cardiovascular events (CV) or worsening of pre-existing CV events have been described in less than 10% of pts, the regimen has an accettable safety and tolerability. Since its approval in Italy, data on real life KRd use are still lacking.
Aims
We conducted an analysis of RRMM pts treated with KRd out of clinical trials to provide further insights on efficacy, tolerability and safety.
Methods
From November 2016 up to now 35 RRMM pts were exposed to KRd. Median number of prior therapies was 2 (range 1-6); 16 (45%) was classified as high risk pts (r-ISS) and 17 (48%) were previously autotransplanted.
Results
We report results of the first 12-months safety interim analysis on KRD treatment of RRMM. Data cut-off was January 2018. 35 pts with a median age of 65 years (range 50-79) were evaluated. All the pts received almost 2 cycles, 1 pts 16 cycles, 2 pts 15 cycles, 3 pts 14 cycles, 2 pts 13 cycles, 2 pts 12 cycles, 3 pts 11 cycles, 2 pts 10 and 9 cycles, 5 pts 8 cycles, 2 pts 7, 6, 5 and 4, 2 pts only 3 cycles, 3 pts only 2 cycles, At least 5 KRD treatment cycles were given to 28 (80%) pts. All pts had a response to treatment at the first/second cycle. At the end of the second cycle 26 (74%) pts obtained a response. Overall response rate with KRD was nearly 80% among the 28 pts that have completed 5 cycles.The majority of the pts(n=26, 96%) responded after at least 1 cycle of chemotherapy. Partial response were observed in 6 pts(21%). Rate of very good PR was 32% (9 pts); 3 pts(10%) achieved complete response (CR), 1 patient minimal response (MR), 6 pts obtained a stable disease, 6 pts obtained a stable response and then progressed. To identify pts that could obtain the most advantage by KRd treatment, we distinguished pts that have completed almost three cycles in two groups, based on previous exposure or not to lenalidomide (Table1). The 13 pts previously treated with lenalidomide (group A), compared to 15 pts not exposed to lenalidomide (group B), showed a worst profile: in the group A there was a higher % of refractory pts(25% vs 11%, p=0.0007) who received more prior treatments, despite the duration of the last treatment was similar in both groups. The regimen was well tolerated. with grade 3-4 haematological and non-haematological AEs on 10 (35%) and 15 (53%) pts respectively. Grade 3-4 non- haematological AEs occurred in 53% of patients, the most common being pneumonia/fever (3/2, 10/7%), hypertension (8, 28%) hiperglicemia (1, 3%), and cardiac failure (2, 7%). Cardiac AEs were 35% (10 pts). Pts with ≥1 CV risk factor at enrolment had a an increased risk of developing a CV AE during treatment as compared to pts with no CV risk factors.1 pt died of infection (not treatment-related). In pts who developed serious AE, KRD dose reduction (2, 7%) and discontinuation (3, 10%) were applied. Onset of CV events significantly increased the rate of dose reductions and treatment discontinuation. CV AEs may significantly impact treatment compliance and survival.

Conclusion
Rate of VGPR obtained with KRd combination was high with an overall response of about 80%. Safety profile was acceptable with a 35% of CV events. Additional analyses are needed to evaluate the impact of these patterns on efficacy outcomes.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Myeloma, Relapse


