
Contributions
Abstract: PB2157
Type: Publication Only
Background
The introduction of novel agents including immunomodulators and proteasome inhibitors has led to an improvement in response rates and survival outcomes in patients with newly diagnosed multiple myeloma. Despite these therapeutic advances a subset of patients do not respond to first-line treatment and biomarkers are needed to identify those at risk.
Aims
To evaluate whether early paraprotein reassessments can predict response to first-line treatment and identify patients at risk for treatment failure.
Methods
We studied 419 patients with newly diagnosed multiple myeloma who were treated in routine clinical practice (n=225, training cohort) or on prospective clinical trial protocols (n=194, validation cohort) between 12/2003 and 12/2015 at Mayo Clinic. All patients had serum M-spike and free light chain (FLC) measurements performed before and after the first treatment cycle. Response to first-line treatment was evaluated using the International Myeloma Working Group Uniform Response Criteria. The biomarkers of interest were the relative decrease in M-spike and absolute FLC difference (ΔFLC). The measurements were standardized to a 28-day interval (divided by the number of days between reassessments and multiplied by 28). Three milestones were defined: Achieving neither a 25% reduction in M-spike nor a 25% reduction in ΔFLC within one treatment cycle (M0), achieving a 25% or greater reduction in at least one of the two biomarkers (M1), and achieving a 25% or greater reduction in both biomarkers (M2). Receiver operator characteristics (ROC) analysis was performed to determine the performance characteristics of these milestones in regards to predicting the best response to first-line treatment.
Results
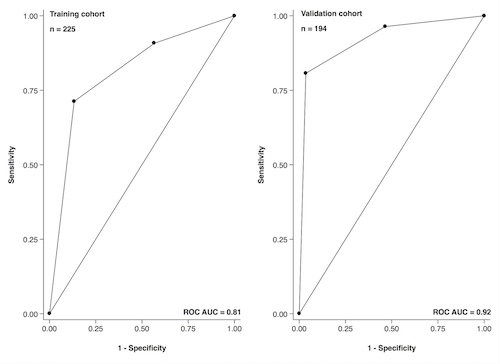
The median age at diagnosis in the training and the validation cohort was 66 (32-94) and 66 years (38-85), respectively. One hundred forty-nine (61%) and 103 patients (53%) were male, respectively. The three most common regimens in the training cohort were lenalidomide + dexamethasone, bortezomib + cyclophosphamide + dexamethasone, and bortezomib + lenalidomide + dexamethasone. The three most common regimens in the validation cohort were carfilzomib + thalidomide + cyclophosphamide, lenalidomide + cyclophosphamide + dexamethasone, and ixazomib + cyclophosphamide + dexamethasone. One hundred ninety-five patients in the training (87%) and 166 patients in the validation cohort (86%) achieved a partial response or better to first-line treatment (PR+). One hundred thirty-nine of the 143 patients (97%) in the training cohort and 134 of the 135 patients (99%) in validation cohort who reached M2 achieved PR+ later on. Thirty-eight of the 51 patients (75%) and 26 of the 38 patients (68%) who reached M1 achieved PR+ later on. Eighteen of the 31 patients (58%) and 6 of the 21 patients (29%) who reached M0 achieved PR+ later on. The ROC curves for the milestones in regards to achieving PR+ are shown in Figure 1. Reaching M2 was fairly sensitive (71%, 95% CI 64-78 and 81%, 95% CI 74-86) and highly specific (87%, 95% CI 69-96 and 96%, 95% CI 82-100) for achieving PR+, translating into a high positive predictive value (97%, 95% CI 93-99 and 99%, 95% CI 96-100).
Conclusion
Patients with newly diagnosed multiple myeloma treated in routine clinical practice and on clinical trial protocols were highly likely to respond to treatment if they experienced at least a 25% decrease in serum M-spike and ΔFLC during the first treatment cycle. Biomarker reassessment after the first treatment cycle may help to identify patients at risk for treatment failure early on.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Immunoglobulin, Multiple Myeloma, Prediction, Serum free light chains
Abstract: PB2157
Type: Publication Only
Background
The introduction of novel agents including immunomodulators and proteasome inhibitors has led to an improvement in response rates and survival outcomes in patients with newly diagnosed multiple myeloma. Despite these therapeutic advances a subset of patients do not respond to first-line treatment and biomarkers are needed to identify those at risk.
Aims
To evaluate whether early paraprotein reassessments can predict response to first-line treatment and identify patients at risk for treatment failure.
Methods
We studied 419 patients with newly diagnosed multiple myeloma who were treated in routine clinical practice (n=225, training cohort) or on prospective clinical trial protocols (n=194, validation cohort) between 12/2003 and 12/2015 at Mayo Clinic. All patients had serum M-spike and free light chain (FLC) measurements performed before and after the first treatment cycle. Response to first-line treatment was evaluated using the International Myeloma Working Group Uniform Response Criteria. The biomarkers of interest were the relative decrease in M-spike and absolute FLC difference (ΔFLC). The measurements were standardized to a 28-day interval (divided by the number of days between reassessments and multiplied by 28). Three milestones were defined: Achieving neither a 25% reduction in M-spike nor a 25% reduction in ΔFLC within one treatment cycle (M0), achieving a 25% or greater reduction in at least one of the two biomarkers (M1), and achieving a 25% or greater reduction in both biomarkers (M2). Receiver operator characteristics (ROC) analysis was performed to determine the performance characteristics of these milestones in regards to predicting the best response to first-line treatment.
Results
The median age at diagnosis in the training and the validation cohort was 66 (32-94) and 66 years (38-85), respectively. One hundred forty-nine (61%) and 103 patients (53%) were male, respectively. The three most common regimens in the training cohort were lenalidomide + dexamethasone, bortezomib + cyclophosphamide + dexamethasone, and bortezomib + lenalidomide + dexamethasone. The three most common regimens in the validation cohort were carfilzomib + thalidomide + cyclophosphamide, lenalidomide + cyclophosphamide + dexamethasone, and ixazomib + cyclophosphamide + dexamethasone. One hundred ninety-five patients in the training (87%) and 166 patients in the validation cohort (86%) achieved a partial response or better to first-line treatment (PR+). One hundred thirty-nine of the 143 patients (97%) in the training cohort and 134 of the 135 patients (99%) in validation cohort who reached M2 achieved PR+ later on. Thirty-eight of the 51 patients (75%) and 26 of the 38 patients (68%) who reached M1 achieved PR+ later on. Eighteen of the 31 patients (58%) and 6 of the 21 patients (29%) who reached M0 achieved PR+ later on. The ROC curves for the milestones in regards to achieving PR+ are shown in Figure 1. Reaching M2 was fairly sensitive (71%, 95% CI 64-78 and 81%, 95% CI 74-86) and highly specific (87%, 95% CI 69-96 and 96%, 95% CI 82-100) for achieving PR+, translating into a high positive predictive value (97%, 95% CI 93-99 and 99%, 95% CI 96-100).

Conclusion
Patients with newly diagnosed multiple myeloma treated in routine clinical practice and on clinical trial protocols were highly likely to respond to treatment if they experienced at least a 25% decrease in serum M-spike and ΔFLC during the first treatment cycle. Biomarker reassessment after the first treatment cycle may help to identify patients at risk for treatment failure early on.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Immunoglobulin, Multiple Myeloma, Prediction, Serum free light chains


