
Contributions
Abstract: PB2159
Type: Publication Only
Background
Despite recent improved survival with the use of novel agents in myeloma, elderly patients have benefited less than younger patients. The median survival for patients age >75 in previous studies is 24-27 months and patients who were untreated survived only 2 months median. Our unit pursues an active policy of offering anti-myeloma treatment with novel agents to all patients regardless of age, performance status (PS) and comorbidity, provided some reversibility is likely. Treatment doses are reduced for those age >75 for the first 1-2 cycles and dosage subsequently escalated if tolerated. We audited the outcome.
Aims
This study aims at evaluating the outcome of the elderly myeloma cohort who were mainly treated with novel agents
Methods
All patients age ≥75 years with symptomatic myeloma presenting to a general hospital from January 2011-June 2017 were included. The following were collected: baseline demographics, ECOG PS, age-adjusted Charlson comorbidity index (aCCI), International Staging System (ISS). Data were analysed using SPSS 20 software and Kaplan-Meier survival curves plotted.
Results
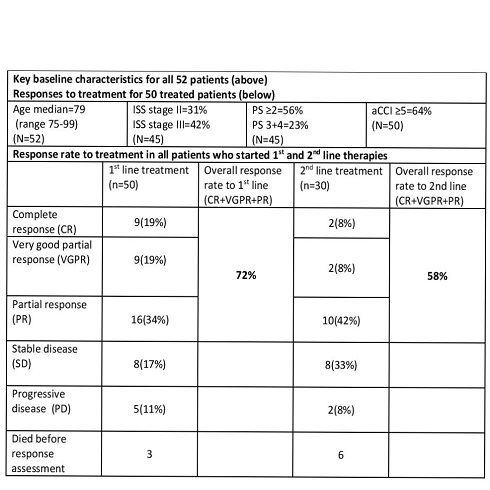
52 patients were found age 75-99 (median 79 years) PS ≥2=56%, aCCI 3-8 (median 5), ISS 3=42%. 50 (96%) patients received therapy and only 2 patients were not treated (1 declined, 1 severe dementia). Of the 50 patients who received first line therapy, 49 were treated with novel agents (bortezomib based=36(72%), thalidomide based=12(24%), lenalidomide based=1(2%)) and 1(2%) with dexamethasone only. 72% achieved a partial response (PR) or better. 8 patients died during treatment (toxicity 1, disease progression 4, unrelated causes 3).
30 patients received second line treatment and the mean time to commence second line treatment (from the commencement of first line treatment) was 16.3 months. All patients were treated with novel agents (43% proteasome inhibitor based, 30% thalidomide based, 26% lenalidomide based). 13 patients underwent third line treatment or more.
At the data cut-off date 24/11/2017 62% (32) patients were still alive. The median overall survival (OS) for the cohort was 45.4 months (95% CI 37-57). Of the 20 patients who died during the study period, the majority (16, 80%) died of disease progression while only 2 died due to treatment related toxicity (the other 2 patients died of unrelated causes). The 3 patients with advanced dementia fared poorly and did not complete treatment. Mild dementia (6) was manageable with support.
Conclusion
Our OS for this group aged ≥75 is better than that reported from the Mayo clinic at 27 months. They reported a similar study from 1999-2008 where only 39% patients received novel agents. Our more recent cohort was treated almost exclusively with novel
agents and this may have contributed to better OS. Our patients were a poor prognosis group in view of their age, PS, ISS and aCCI. Treatment was therefore dose reduced from the start and this may have contributed to the small number of toxic deaths (2) and the overall tolerability of treatment with few discontinuations. Despite the dose reductions, patients gained useful responses and OS was encouraging.
Although significant numbers of patients had poor prognostic factors (PS, aCCI, ISS stage) in this group, we have shown that the majority of patients tolerated treatment and had a positive response. We observed more than double the OS in our cohort compared to those published in the literature (45.4 months vs 22 months). In conclusion, an active treatment strategy regardless of age and comorbidity can benefit elderly patients.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Comorbidities, Elderly, Outcome, Survival
Abstract: PB2159
Type: Publication Only
Background
Despite recent improved survival with the use of novel agents in myeloma, elderly patients have benefited less than younger patients. The median survival for patients age >75 in previous studies is 24-27 months and patients who were untreated survived only 2 months median. Our unit pursues an active policy of offering anti-myeloma treatment with novel agents to all patients regardless of age, performance status (PS) and comorbidity, provided some reversibility is likely. Treatment doses are reduced for those age >75 for the first 1-2 cycles and dosage subsequently escalated if tolerated. We audited the outcome.
Aims
This study aims at evaluating the outcome of the elderly myeloma cohort who were mainly treated with novel agents
Methods
All patients age ≥75 years with symptomatic myeloma presenting to a general hospital from January 2011-June 2017 were included. The following were collected: baseline demographics, ECOG PS, age-adjusted Charlson comorbidity index (aCCI), International Staging System (ISS). Data were analysed using SPSS 20 software and Kaplan-Meier survival curves plotted.
Results
52 patients were found age 75-99 (median 79 years) PS ≥2=56%, aCCI 3-8 (median 5), ISS 3=42%. 50 (96%) patients received therapy and only 2 patients were not treated (1 declined, 1 severe dementia). Of the 50 patients who received first line therapy, 49 were treated with novel agents (bortezomib based=36(72%), thalidomide based=12(24%), lenalidomide based=1(2%)) and 1(2%) with dexamethasone only. 72% achieved a partial response (PR) or better. 8 patients died during treatment (toxicity 1, disease progression 4, unrelated causes 3).
30 patients received second line treatment and the mean time to commence second line treatment (from the commencement of first line treatment) was 16.3 months. All patients were treated with novel agents (43% proteasome inhibitor based, 30% thalidomide based, 26% lenalidomide based). 13 patients underwent third line treatment or more.
At the data cut-off date 24/11/2017 62% (32) patients were still alive. The median overall survival (OS) for the cohort was 45.4 months (95% CI 37-57). Of the 20 patients who died during the study period, the majority (16, 80%) died of disease progression while only 2 died due to treatment related toxicity (the other 2 patients died of unrelated causes). The 3 patients with advanced dementia fared poorly and did not complete treatment. Mild dementia (6) was manageable with support.

Conclusion
Our OS for this group aged ≥75 is better than that reported from the Mayo clinic at 27 months. They reported a similar study from 1999-2008 where only 39% patients received novel agents. Our more recent cohort was treated almost exclusively with novel
agents and this may have contributed to better OS. Our patients were a poor prognosis group in view of their age, PS, ISS and aCCI. Treatment was therefore dose reduced from the start and this may have contributed to the small number of toxic deaths (2) and the overall tolerability of treatment with few discontinuations. Despite the dose reductions, patients gained useful responses and OS was encouraging.
Although significant numbers of patients had poor prognostic factors (PS, aCCI, ISS stage) in this group, we have shown that the majority of patients tolerated treatment and had a positive response. We observed more than double the OS in our cohort compared to those published in the literature (45.4 months vs 22 months). In conclusion, an active treatment strategy regardless of age and comorbidity can benefit elderly patients.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Comorbidities, Elderly, Outcome, Survival


