
Contributions
Abstract: PB2213
Type: Publication Only
Background
The progression-free and overall survival are key indicators that reflect the effective therapy in patients with hematological disease. The use of different prognostic models based on various factors and their combinations allows to determine the optimal strategy for treating patients, including patients with multiple myeloma (MM) and improve survival. There are quantitative factors and qualitative signs which are determined before and after the induction therapy, and after autologous stem cells transplantation (AutoSCT), if this option was available. However, the advantage of each prognostic parameter should be revised when using new drugs and their combinations.
Aims
To define the factors influencing on progression-free survival (PFS) by means of one-factor and multifactor analyses.
Methods
We analyzed 72 patients with MM (median age 59 years, male/female – 1.25:1). The induction therapy with Bortezomib-based regimens (VD, CVD, VMP, PAD) was used in 48/72 (66.7%) patients, Immunomodulator-based regimens (Thal+D, RD, VRD, PomD) – in 20/72 (27.8%), chemotherapy – in 4/72 (5.5%). Autologous stem cell transplantation (ASCT) is carried out 48 (66.7%) patients. We used the sex, age, the variant induction antimyeloma therapy, response after treatment, MFC MRD status, PET-CT status, tumor load, AutoSCT, variant of maintenance therapy as prognostic factors. The MFC MRD status and PET-CT status were evaluated after 4-6 cycles of induction therapy and after ASCT, if it (AutoSCT) was performed. For definition of MRD we used 5-color flow cytometry with CD38, CD138, CD45, CD19, CD20, CD27, CD56 and CD117 antibodies. The MFC MRD– (<10-4) response was stated at identification less than 0.01% of clonal plasma cells and MFC MRD– (<10-5) – less than 0.001%. The PET-CT was done in 30 patients only. The PET-CT– status was established if specific accumulation of 18-FDG was absent.
Results
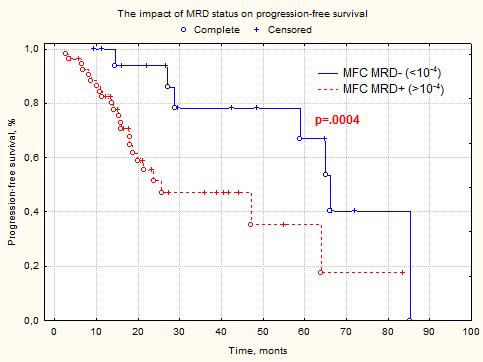
The general regression models (one-factor analysis) show reliable influence of complete response (CR) (р=.0004) and tumor load in bone marrow (р=.000006) on PFS. The multifactor analysis among these parameters shows the CR above the non-CR (VGPR, PR, SD), MFC MRD– status (MRD <10-4) above MFC MRD+ (p<0.05). Simultaneous use of factors (achievement of CR and MRD <10-4) show reliable impact on PFS (р=.0006). The MFC MRD– (<10-4) was reached in 25% (18/72), The MFC MRD– (<10-5) – in 9.7% (7/72). The PFS median in MFC MRD+ (>10-4) group was 23 months, in the MFC MRD– (<10-4) was 65 months (p=.0004). The reliable MFC MRD– (<10-5) above MFC MRD– (<10-4) and MFC MRD+ (>10-5) was not achieved (р>.05). The PFS median in MFC MRD– (<10-5) group was 67 months, in the MFC MRD+ (>10-5) was 47 months (p>.05). It is probably caused that small number of patients with MFC MRD– (<10-5) and short period of observation. The sex, age, variant of induction antimyeloma, PET-CT status, AutoSCT and variant of maintenance therapy were not impact on PFS (р>.05).
Conclusion
The presence of MFC MRD is unfavorable prognostic factor. Achievement CR and MRD– (<10-4) status are favorably influence on duration of PFS in MM patients.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Minimal residual disease (MRD), Myeloma, Prognostic factor, Survival
Abstract: PB2213
Type: Publication Only
Background
The progression-free and overall survival are key indicators that reflect the effective therapy in patients with hematological disease. The use of different prognostic models based on various factors and their combinations allows to determine the optimal strategy for treating patients, including patients with multiple myeloma (MM) and improve survival. There are quantitative factors and qualitative signs which are determined before and after the induction therapy, and after autologous stem cells transplantation (AutoSCT), if this option was available. However, the advantage of each prognostic parameter should be revised when using new drugs and their combinations.
Aims
To define the factors influencing on progression-free survival (PFS) by means of one-factor and multifactor analyses.
Methods
We analyzed 72 patients with MM (median age 59 years, male/female – 1.25:1). The induction therapy with Bortezomib-based regimens (VD, CVD, VMP, PAD) was used in 48/72 (66.7%) patients, Immunomodulator-based regimens (Thal+D, RD, VRD, PomD) – in 20/72 (27.8%), chemotherapy – in 4/72 (5.5%). Autologous stem cell transplantation (ASCT) is carried out 48 (66.7%) patients. We used the sex, age, the variant induction antimyeloma therapy, response after treatment, MFC MRD status, PET-CT status, tumor load, AutoSCT, variant of maintenance therapy as prognostic factors. The MFC MRD status and PET-CT status were evaluated after 4-6 cycles of induction therapy and after ASCT, if it (AutoSCT) was performed. For definition of MRD we used 5-color flow cytometry with CD38, CD138, CD45, CD19, CD20, CD27, CD56 and CD117 antibodies. The MFC MRD– (<10-4) response was stated at identification less than 0.01% of clonal plasma cells and MFC MRD– (<10-5) – less than 0.001%. The PET-CT was done in 30 patients only. The PET-CT– status was established if specific accumulation of 18-FDG was absent.
Results
The general regression models (one-factor analysis) show reliable influence of complete response (CR) (р=.0004) and tumor load in bone marrow (р=.000006) on PFS. The multifactor analysis among these parameters shows the CR above the non-CR (VGPR, PR, SD), MFC MRD– status (MRD <10-4) above MFC MRD+ (p<0.05). Simultaneous use of factors (achievement of CR and MRD <10-4) show reliable impact on PFS (р=.0006). The MFC MRD– (<10-4) was reached in 25% (18/72), The MFC MRD– (<10-5) – in 9.7% (7/72). The PFS median in MFC MRD+ (>10-4) group was 23 months, in the MFC MRD– (<10-4) was 65 months (p=.0004). The reliable MFC MRD– (<10-5) above MFC MRD– (<10-4) and MFC MRD+ (>10-5) was not achieved (р>.05). The PFS median in MFC MRD– (<10-5) group was 67 months, in the MFC MRD+ (>10-5) was 47 months (p>.05). It is probably caused that small number of patients with MFC MRD– (<10-5) and short period of observation. The sex, age, variant of induction antimyeloma, PET-CT status, AutoSCT and variant of maintenance therapy were not impact on PFS (р>.05).

Conclusion
The presence of MFC MRD is unfavorable prognostic factor. Achievement CR and MRD– (<10-4) status are favorably influence on duration of PFS in MM patients.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Minimal residual disease (MRD), Myeloma, Prognostic factor, Survival


