
Contributions
Abstract: PB2130
Type: Publication Only
Background
Mesangiogenic Progenitor Cells (MPCs) are a bone marrow (BM) cell population isolated in humans able to differentiate into mesenchymal stromal cells (MSCs) and retaining an angiogenic potential. These two differentiation fates are mutually-exclusive, MPC-derived MSCs are not able to take part in angiogenic process but they can differentiate into adipocytes, chondroblasts or osteoblasts. The MPC differentiation toward the mesengenic lineage has been demonstrated to activate the non-canonical Wnt pathway, not involved during angiogenesis. More specifically, the MSCs differentiation takes place toward two hierarchical steps: a first differentiation into “early MSCs” (also called P1-MSCs) with the activation of Wnt-5/calmodulin pathway, then a terminal differentiation into “late MSCs” (also called P2-MSCs) independent from this pathway. Indeed, it has been demonstrated that Calmidazolium Chloride (CLMDZ), a potent calmodulin inhibitor, blocks the MPC mesengenic differentiation acting on the early phase. Previous studies, conducted on BM samples of non-hematological patients, demonstrated that the MPC angiogenic differentiation is, instead, inhibited by Bortezomib. For these peculiar characteristics, MPCs can be thought to be involved in the pathogenesis and progression of Multiple Myeloma (MM).
The same in vitro experiments performed on BM samples from newly diagnosed MM patients, surprisingly showed that both mesengenic and angiogenic differentiations were impaired by Bortezomib while CLMDZ did not affect any differentiation. This data suggests that possibly MPCs would be restricted to an angiogenic fate, losing the mesengenic potential in the pathological setting.
Aims
To evaluate a possible involvement of MPCs in MM pathogenesis, we assessed the angiogenic potential of P1-MSCs applying sprouting tests.
Methods
After written consent, BM samples were obtained from 11 newly diagnosed MM patients. We isolated MPCs, as previously described, from each sample and performed mesengenic differentiation applying a specific medium for MSC expansion. After six days of culture, P1-MSCs were detached and two 3D-spheroids were produced by the hanging drop method. The spheroids were then plated on Matrigel thick gel and cultured in EGM-2 endothelial growth medium for one week. Sprouting distance was then measured by image analysis software and the mean values obtained from three different observers were recorded and analyzed by t-test.
Results
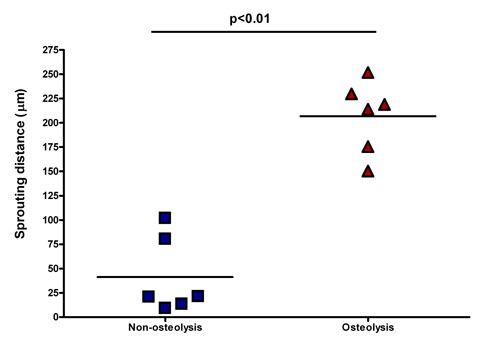
Six of eleven patients have a mean sprouting value of 41.2±16.1μm while the other five have a mean value of 206.7±15.2μm, the statistical analysis demonstrates a significative difference (p<0.01) between these two groups (figure).
Surprisingly, higher sprouting values have been detected into patients with a more aggressive stage of disease and reporting osteolytic lesions, instead, a reduced sprouting has been detected in patients without osteolytic lesions or affected by smoldering MM.
Conclusion
Multiple Myeloma as a BM niche disease, affects the microenvironment. Particularly, our results suggest that disease in advanced stage (characterized by osteolytic lesions) probably forces MPCs toward an angiogenic differentiation regardless of the culture conditions. Thus, MPCs could be involved in the transition from a sub-clinical and “non-vascular” stage of disease to the advanced stages. Moreover, this MPCs restriction to the angiogenic fate could correlate in vivo with a reduced mesengenesis which cooperates to osteolytic lesions.
Session topic: 13. Myeloma and other monoclonal gammopathies – Biology & Translational Research
Keyword(s): Angiogenesis, Bone disease, Mesenchymal stem cell, Multiple Myeloma
Abstract: PB2130
Type: Publication Only
Background
Mesangiogenic Progenitor Cells (MPCs) are a bone marrow (BM) cell population isolated in humans able to differentiate into mesenchymal stromal cells (MSCs) and retaining an angiogenic potential. These two differentiation fates are mutually-exclusive, MPC-derived MSCs are not able to take part in angiogenic process but they can differentiate into adipocytes, chondroblasts or osteoblasts. The MPC differentiation toward the mesengenic lineage has been demonstrated to activate the non-canonical Wnt pathway, not involved during angiogenesis. More specifically, the MSCs differentiation takes place toward two hierarchical steps: a first differentiation into “early MSCs” (also called P1-MSCs) with the activation of Wnt-5/calmodulin pathway, then a terminal differentiation into “late MSCs” (also called P2-MSCs) independent from this pathway. Indeed, it has been demonstrated that Calmidazolium Chloride (CLMDZ), a potent calmodulin inhibitor, blocks the MPC mesengenic differentiation acting on the early phase. Previous studies, conducted on BM samples of non-hematological patients, demonstrated that the MPC angiogenic differentiation is, instead, inhibited by Bortezomib. For these peculiar characteristics, MPCs can be thought to be involved in the pathogenesis and progression of Multiple Myeloma (MM).
The same in vitro experiments performed on BM samples from newly diagnosed MM patients, surprisingly showed that both mesengenic and angiogenic differentiations were impaired by Bortezomib while CLMDZ did not affect any differentiation. This data suggests that possibly MPCs would be restricted to an angiogenic fate, losing the mesengenic potential in the pathological setting.
Aims
To evaluate a possible involvement of MPCs in MM pathogenesis, we assessed the angiogenic potential of P1-MSCs applying sprouting tests.
Methods
After written consent, BM samples were obtained from 11 newly diagnosed MM patients. We isolated MPCs, as previously described, from each sample and performed mesengenic differentiation applying a specific medium for MSC expansion. After six days of culture, P1-MSCs were detached and two 3D-spheroids were produced by the hanging drop method. The spheroids were then plated on Matrigel thick gel and cultured in EGM-2 endothelial growth medium for one week. Sprouting distance was then measured by image analysis software and the mean values obtained from three different observers were recorded and analyzed by t-test.
Results
Six of eleven patients have a mean sprouting value of 41.2±16.1μm while the other five have a mean value of 206.7±15.2μm, the statistical analysis demonstrates a significative difference (p<0.01) between these two groups (figure).
Surprisingly, higher sprouting values have been detected into patients with a more aggressive stage of disease and reporting osteolytic lesions, instead, a reduced sprouting has been detected in patients without osteolytic lesions or affected by smoldering MM.

Conclusion
Multiple Myeloma as a BM niche disease, affects the microenvironment. Particularly, our results suggest that disease in advanced stage (characterized by osteolytic lesions) probably forces MPCs toward an angiogenic differentiation regardless of the culture conditions. Thus, MPCs could be involved in the transition from a sub-clinical and “non-vascular” stage of disease to the advanced stages. Moreover, this MPCs restriction to the angiogenic fate could correlate in vivo with a reduced mesengenesis which cooperates to osteolytic lesions.
Session topic: 13. Myeloma and other monoclonal gammopathies – Biology & Translational Research
Keyword(s): Angiogenesis, Bone disease, Mesenchymal stem cell, Multiple Myeloma


