
Contributions
Abstract: PB2117
Type: Publication Only
Background
Assessment of MYD88 p.L265P mutation has been implemented in clinical routine as a diagnostic tool in IgM monoclonal gammopathy. Although bone marrow sample is usually available at diagnosis, the study of peripheral blood is useful for patient follow-up or in absence of bone marrow sample. However, the proportion of cells with the mutation in peripheral blood may be very low, which makes it difficult to detect them, leading to a false negative result. A strategy to overcome this situation is to enrich the target population and to use high sensitivity techniques. Enrichment of the sample in mutated cells can be done by depletion of CD3-positive lymphocytes (as the expression of CD19 is usually weak in the B-cell clonal population). Digital PCR is a technology that allows the detection and quantification of mutated DNA, even when it is present in a low proportion of total DNA.
Aims
To analyze the mutational status of MYD88 p.L265P in peripheral blood samples by real-time allele-specific PCR (AS-qPCR) and digital PCR in a series of patients with IgM monoclonal gammopathy, as well as to determine the utility of the enrichment of the CD3-negative population for the detection of this mutation.
Methods
41 peripheral blood samples were collected from 36 patients with IgM monoclonal gammopathy (28 with Waldenström macroglobulinemia (WM) and 8 with IgM monoclonal gammopathy of undetermined significance (IgM MGUS)). Depletion of CD3-positive lymphocytes was performed and DNA was obtained from the fraction of total mononuclear cells and from the CD3-negative subfraction. The MYD88 p.L265P mutation was analyzed by AS-qPCR (7500Fast, Applied Biosystems) and digital PCR (QuantStudio 3D, Applied Biosystems).
Results
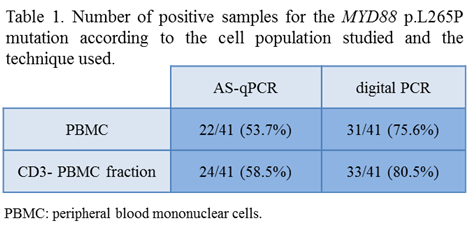
When the total mononuclear cell fraction was analyzed, MYD88 p.L265P was detected in 22 from the 41 samples studied (53.65%) using AS-qPCR and in 31/41 samples (75.6%) by digital PCR. The analysis of the CD3-negative fraction allowed the detection of the MYD88 p.L265P in 24/41 samples (58.5%) by AS-qPCR and in 33/41 (80.5%) by digital PCR. These results are summarized in Table 1. In the CD3-negative fraction, the average mutational load was of 1.72% (range: 0.05%>16.75%), which was significantly higher than the observed in the total mononuclear cell population: mean 1.13% (range: 0.05%>11.21%), p=0.004. The negative cases by AS-qPCR corresponded to cases with low mutational load (<0.25%). No significant differences were observed in the allelic load between WM and IgM MGUS cases. As a whole, MYD88 p.L265P was detected in 29 from the 36 cases studied, 6/8 (75%) IgM MGUS and 23/28 (82%) WM patients. This later percentage rose up to 91% (21/23) when only untreated WM patients were considered.
Conclusion
In patients with MW and IgM MGUS, the application of high sensitivity techniques such as digital PCR and, to a lesser extent, the enrichment of the sample in tumoral cells by T-cell depletion, improve the detection of MYD88 p.L265P mutation.
Session topic: 13. Myeloma and other monoclonal gammopathies – Biology & Translational Research
Keyword(s): MGUS, mutation analysis, Waldenstrom's macroglobulinemia
Abstract: PB2117
Type: Publication Only
Background
Assessment of MYD88 p.L265P mutation has been implemented in clinical routine as a diagnostic tool in IgM monoclonal gammopathy. Although bone marrow sample is usually available at diagnosis, the study of peripheral blood is useful for patient follow-up or in absence of bone marrow sample. However, the proportion of cells with the mutation in peripheral blood may be very low, which makes it difficult to detect them, leading to a false negative result. A strategy to overcome this situation is to enrich the target population and to use high sensitivity techniques. Enrichment of the sample in mutated cells can be done by depletion of CD3-positive lymphocytes (as the expression of CD19 is usually weak in the B-cell clonal population). Digital PCR is a technology that allows the detection and quantification of mutated DNA, even when it is present in a low proportion of total DNA.
Aims
To analyze the mutational status of MYD88 p.L265P in peripheral blood samples by real-time allele-specific PCR (AS-qPCR) and digital PCR in a series of patients with IgM monoclonal gammopathy, as well as to determine the utility of the enrichment of the CD3-negative population for the detection of this mutation.
Methods
41 peripheral blood samples were collected from 36 patients with IgM monoclonal gammopathy (28 with Waldenström macroglobulinemia (WM) and 8 with IgM monoclonal gammopathy of undetermined significance (IgM MGUS)). Depletion of CD3-positive lymphocytes was performed and DNA was obtained from the fraction of total mononuclear cells and from the CD3-negative subfraction. The MYD88 p.L265P mutation was analyzed by AS-qPCR (7500Fast, Applied Biosystems) and digital PCR (QuantStudio 3D, Applied Biosystems).
Results
When the total mononuclear cell fraction was analyzed, MYD88 p.L265P was detected in 22 from the 41 samples studied (53.65%) using AS-qPCR and in 31/41 samples (75.6%) by digital PCR. The analysis of the CD3-negative fraction allowed the detection of the MYD88 p.L265P in 24/41 samples (58.5%) by AS-qPCR and in 33/41 (80.5%) by digital PCR. These results are summarized in Table 1. In the CD3-negative fraction, the average mutational load was of 1.72% (range: 0.05%>16.75%), which was significantly higher than the observed in the total mononuclear cell population: mean 1.13% (range: 0.05%>11.21%), p=0.004. The negative cases by AS-qPCR corresponded to cases with low mutational load (<0.25%). No significant differences were observed in the allelic load between WM and IgM MGUS cases. As a whole, MYD88 p.L265P was detected in 29 from the 36 cases studied, 6/8 (75%) IgM MGUS and 23/28 (82%) WM patients. This later percentage rose up to 91% (21/23) when only untreated WM patients were considered.

Conclusion
In patients with MW and IgM MGUS, the application of high sensitivity techniques such as digital PCR and, to a lesser extent, the enrichment of the sample in tumoral cells by T-cell depletion, improve the detection of MYD88 p.L265P mutation.
Session topic: 13. Myeloma and other monoclonal gammopathies – Biology & Translational Research
Keyword(s): MGUS, mutation analysis, Waldenstrom's macroglobulinemia


