
Contributions
Abstract: PB2124
Type: Publication Only
Background
Multiple myeloma is an incurable malignancy of plasma cells that develops in the bone marrow (BM). 2D cell line cultures are commonly used to assess the therapeutic potential of experimental anticancer treatments against myeloma in vitro. These 2D cultures do not mimic the dependency of primary myeloma cells on the surrounding BM, nor the BM itself. Since myeloma progression and resistance to therapy are dependent on the BM microenvironment, these essential characteristics need to be taken into account when assessing the therapeutic potential of experimental anticancer interventions.
Aims
Our first aim was to analyze the feasibility of testing novel therapeutics in a previously developed 3D BM myeloma model. For this, both a cellular immunotherapy (αβT cells engineered to express a defined γδTCR; TEGs) and a nanomedicine delivery system (VLA4 targeted liposomal drug delivery) were tested. Secondly, the effect of the therapies on both the cultured myeloma cells and the surrounding BM was analyzed.
Methods
The 3D BM myeloma model components are mesenchymal stromal cells, endothelial progenitor cells and (primary) myeloma cells co-cultured in hydrogel. The 3D BM myeloma model allows non-invasive time-lapse imaging, following the (primary) myeloma cells in the culture system before and after treatment. After 7 or 14 days of pre-culture (allowing myeloma outgrowth), the novel therapeutics were added. The delivery, migration and effect of the therapy were followed for 48 hours. The targeted VLA4 liposomes were loaded with doxorubicin and compared to standard doxorubicin treatment.
Results
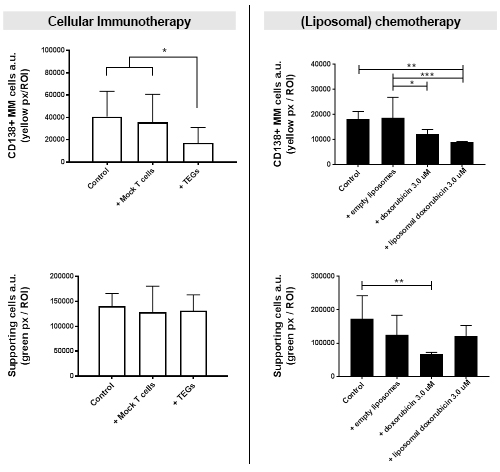
The TEGs, when added to the 3D BM myeloma model, were capable of migrating through the 3D model, exerting a killing response towards the primary myeloma cells in 6 out of 8 donor samples after both 24 and 48 hours. This was not observed when adding non-functional control T cells. The supporting stromal microenvironment was unaffected in all conditions after 48 hours.
The VLA4 targeted liposomes of different sizes (70 nm, 100 nm and 200 nm) were injected in the 3D BM myeloma model. Only the 70 nm and 100 nm liposomes were capable of passively diffusing through the 3D model. Cellular uptake of the liposomes was observed throughout the model, displaying a concentration gradient after injection. When comparing standard doxorubicin therapy to liposomal doxorubicin therapy, similar killing effects towards myeloma cells were observed. However, the targeted liposomal drug therapy induced significantly less unwanted stromal and endothelial cell death compared to the standard doxorubicin therapy.
Conclusion
The previously developed 3D BM myeloma model allows the in vitro study of both cellular immunotherapy and a nanomedicine delivery system on (primary) myeloma cells. Equally important, this model allows testing within an engineered BM environment, analyzing treatment effects on the BM environment as well. The model can thus be used to study cellular targeting throughout the model and general effectiveness of the added therapy by analysis of both on- and off-target effects.
Session topic: 13. Myeloma and other monoclonal gammopathies – Biology & Translational Research
Keyword(s): Bone microenvironment, Multiple Myeloma, Therapy
Abstract: PB2124
Type: Publication Only
Background
Multiple myeloma is an incurable malignancy of plasma cells that develops in the bone marrow (BM). 2D cell line cultures are commonly used to assess the therapeutic potential of experimental anticancer treatments against myeloma in vitro. These 2D cultures do not mimic the dependency of primary myeloma cells on the surrounding BM, nor the BM itself. Since myeloma progression and resistance to therapy are dependent on the BM microenvironment, these essential characteristics need to be taken into account when assessing the therapeutic potential of experimental anticancer interventions.
Aims
Our first aim was to analyze the feasibility of testing novel therapeutics in a previously developed 3D BM myeloma model. For this, both a cellular immunotherapy (αβT cells engineered to express a defined γδTCR; TEGs) and a nanomedicine delivery system (VLA4 targeted liposomal drug delivery) were tested. Secondly, the effect of the therapies on both the cultured myeloma cells and the surrounding BM was analyzed.
Methods
The 3D BM myeloma model components are mesenchymal stromal cells, endothelial progenitor cells and (primary) myeloma cells co-cultured in hydrogel. The 3D BM myeloma model allows non-invasive time-lapse imaging, following the (primary) myeloma cells in the culture system before and after treatment. After 7 or 14 days of pre-culture (allowing myeloma outgrowth), the novel therapeutics were added. The delivery, migration and effect of the therapy were followed for 48 hours. The targeted VLA4 liposomes were loaded with doxorubicin and compared to standard doxorubicin treatment.
Results
The TEGs, when added to the 3D BM myeloma model, were capable of migrating through the 3D model, exerting a killing response towards the primary myeloma cells in 6 out of 8 donor samples after both 24 and 48 hours. This was not observed when adding non-functional control T cells. The supporting stromal microenvironment was unaffected in all conditions after 48 hours.
The VLA4 targeted liposomes of different sizes (70 nm, 100 nm and 200 nm) were injected in the 3D BM myeloma model. Only the 70 nm and 100 nm liposomes were capable of passively diffusing through the 3D model. Cellular uptake of the liposomes was observed throughout the model, displaying a concentration gradient after injection. When comparing standard doxorubicin therapy to liposomal doxorubicin therapy, similar killing effects towards myeloma cells were observed. However, the targeted liposomal drug therapy induced significantly less unwanted stromal and endothelial cell death compared to the standard doxorubicin therapy.

Conclusion
The previously developed 3D BM myeloma model allows the in vitro study of both cellular immunotherapy and a nanomedicine delivery system on (primary) myeloma cells. Equally important, this model allows testing within an engineered BM environment, analyzing treatment effects on the BM environment as well. The model can thus be used to study cellular targeting throughout the model and general effectiveness of the added therapy by analysis of both on- and off-target effects.
Session topic: 13. Myeloma and other monoclonal gammopathies – Biology & Translational Research
Keyword(s): Bone microenvironment, Multiple Myeloma, Therapy


