
Contributions
Abstract: PB1822
Type: Publication Only
Background
Minor populations of glycosylphosphatidylinositol-anchored protein (GPI-AP)-deficient (GPI[-]) cells are often detected in patients with acquired aplastic anemia (AA) or low-risk myelodysplastic syndromes (MDS) and are known to predict a good response to immunosuppressive therapy (IST) and a favorable prognosis. Recently, the Clinical and Laboratory Standards Institute (CLSI) established a high-sensitivity flow cytometry (FCM) assay using fluorescence-labeled aerolysin (FLAER)/CD24/CD15/CD45 (CLSI method) that can detect GPI(-) cells at a sensitivity of 0.01%. We have been using a different high-resolution FCM method that defines ≥0.003% GPI(-) granulocytes and ≥0.005% GPI(-) erythrocytes as an abnormal increase. The principle of this method (OPTIMA method) is based on the elimination of as many false GPI(-) cells that can be detected in healthy individuals as possible. However, whether GPI(-) cells detected by the two different FCM assays identify the same GPI(-) cell population is unclear.
Aims
To compare these two high-sensitivity FCM assays, we performed the clinical study “Comparison of Methods in PNH clone size Assessment by CLSI Recognized or Enhanced high-sensitivity flow cytometry (COMPARE)”, which aimed to determine the percentage of GPI(-) cells in peripheral blood of BM failure (BMF) patients possessing minor populations (0.003% - 0.01%) of GPI(-) cells, and studied the clinical characteristics of these patients.
Methods
We analyzed the peripheral blood of 18 BMF patients (13 AA, 3 MDS, and 2 undifferentiated BMF) who had been judged positive for GPI(-) cells (around 0.01%) by previous analyses using the OPTIMA method. New blood samples were subjected to CLSI and OPTIMA assays on the same day at two different institutions. For 8 of the 18 patients, chronological changes in the GPI(-) clone size were studied over a 3-year period.
Results
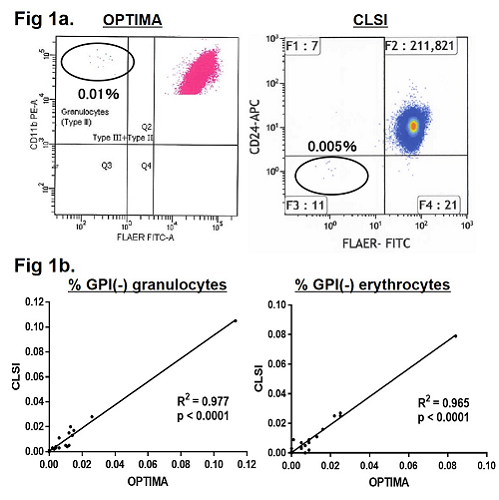
GPI(-) granulocytes≥0.003% were detected in 14 patients (78%) by the OPTIMA method, while GPI(-) granulocytes≥0.01% were detected in 7 patients (39%) by the CLSI method. The OPTIMA method revealed 0.003% - 0.012% GPI(-) granulocytes in 7 patients who were judged negative by the CLSI method because their GPI(-) granulocyte percentages were less than 0.01% (0.002% - 0.005%) (Fig 1a). Four BMF patients who had 0.003% - 0.012% GPI(-) granulocytes in previous OPTIMA analyses were judged GPI(-) cells negative by both methods, due to spontaneous regression of their PNH clones. The median GPI(-) granulocyte clone size of the PNH-positive patients was 0.011% (0.003% - 0.113%) with the OPTIMA method and 0.017% (0.011% - 0.105%) with the CLSI assay. The clone sizes of GPI(-) cells revealed by each assay were positively correlated (Fig. 1b). Of the 14 BMF patients with GPI(-) cells revealed by the OPTIMA, 9 patients (8 AA and 1 MDS-RCMD) received IST (4 with ATG+ CsA and 5 with CsA monotherapy), and all of them achieved partial or complete remission. The GPI(-) clone size of 8 BMF patients (5 AA and 3 MDS) was monitored over a 3-year period, and 0.003% - 0.048% GPI(-) clones were consistently revealed by the OPTIMA method in all cases.
Conclusion
Both high-sensitivity FCM methods produced comparable results in terms of detecting minor GPI(-) cell populations. However, when the CLSI method is used to detect GPI(-) cells in BMF patients, some patients who were actually positive for minor GPI(-) cell populations may be judged negative due to its high cut-off level (0.01%). Thus, caution is needed when <0.01% GPI(-) cells were revealed by the CLSI method; the patients may be positive for increased PNH-type cells and likely to respond to IST.
Session topic: 12. Bone marrow failure syndromes incl. PNH - Clinical
Keyword(s): flow cytometry, PNH
Abstract: PB1822
Type: Publication Only
Background
Minor populations of glycosylphosphatidylinositol-anchored protein (GPI-AP)-deficient (GPI[-]) cells are often detected in patients with acquired aplastic anemia (AA) or low-risk myelodysplastic syndromes (MDS) and are known to predict a good response to immunosuppressive therapy (IST) and a favorable prognosis. Recently, the Clinical and Laboratory Standards Institute (CLSI) established a high-sensitivity flow cytometry (FCM) assay using fluorescence-labeled aerolysin (FLAER)/CD24/CD15/CD45 (CLSI method) that can detect GPI(-) cells at a sensitivity of 0.01%. We have been using a different high-resolution FCM method that defines ≥0.003% GPI(-) granulocytes and ≥0.005% GPI(-) erythrocytes as an abnormal increase. The principle of this method (OPTIMA method) is based on the elimination of as many false GPI(-) cells that can be detected in healthy individuals as possible. However, whether GPI(-) cells detected by the two different FCM assays identify the same GPI(-) cell population is unclear.
Aims
To compare these two high-sensitivity FCM assays, we performed the clinical study “Comparison of Methods in PNH clone size Assessment by CLSI Recognized or Enhanced high-sensitivity flow cytometry (COMPARE)”, which aimed to determine the percentage of GPI(-) cells in peripheral blood of BM failure (BMF) patients possessing minor populations (0.003% - 0.01%) of GPI(-) cells, and studied the clinical characteristics of these patients.
Methods
We analyzed the peripheral blood of 18 BMF patients (13 AA, 3 MDS, and 2 undifferentiated BMF) who had been judged positive for GPI(-) cells (around 0.01%) by previous analyses using the OPTIMA method. New blood samples were subjected to CLSI and OPTIMA assays on the same day at two different institutions. For 8 of the 18 patients, chronological changes in the GPI(-) clone size were studied over a 3-year period.
Results
GPI(-) granulocytes≥0.003% were detected in 14 patients (78%) by the OPTIMA method, while GPI(-) granulocytes≥0.01% were detected in 7 patients (39%) by the CLSI method. The OPTIMA method revealed 0.003% - 0.012% GPI(-) granulocytes in 7 patients who were judged negative by the CLSI method because their GPI(-) granulocyte percentages were less than 0.01% (0.002% - 0.005%) (Fig 1a). Four BMF patients who had 0.003% - 0.012% GPI(-) granulocytes in previous OPTIMA analyses were judged GPI(-) cells negative by both methods, due to spontaneous regression of their PNH clones. The median GPI(-) granulocyte clone size of the PNH-positive patients was 0.011% (0.003% - 0.113%) with the OPTIMA method and 0.017% (0.011% - 0.105%) with the CLSI assay. The clone sizes of GPI(-) cells revealed by each assay were positively correlated (Fig. 1b). Of the 14 BMF patients with GPI(-) cells revealed by the OPTIMA, 9 patients (8 AA and 1 MDS-RCMD) received IST (4 with ATG+ CsA and 5 with CsA monotherapy), and all of them achieved partial or complete remission. The GPI(-) clone size of 8 BMF patients (5 AA and 3 MDS) was monitored over a 3-year period, and 0.003% - 0.048% GPI(-) clones were consistently revealed by the OPTIMA method in all cases.

Conclusion
Both high-sensitivity FCM methods produced comparable results in terms of detecting minor GPI(-) cell populations. However, when the CLSI method is used to detect GPI(-) cells in BMF patients, some patients who were actually positive for minor GPI(-) cell populations may be judged negative due to its high cut-off level (0.01%). Thus, caution is needed when <0.01% GPI(-) cells were revealed by the CLSI method; the patients may be positive for increased PNH-type cells and likely to respond to IST.
Session topic: 12. Bone marrow failure syndromes incl. PNH - Clinical
Keyword(s): flow cytometry, PNH


