
Contributions
Abstract: PB1821
Type: Publication Only
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare hematopoietic stem cell disorder acquired by somatic mutation of phosphatidylinositol glycan–class A (PIG-A) gene. PIG-A mutation leads partial or absolute defect in expression of GPI-anchored cell surface structure, and this result in episodes of intravascular hemolysis that are typical of the disease. Flow cytometric techniques with utilizing the FLAER enable to sensitive identify very small numbers of GPI-deficient cells. Flow cytometric PNH testing uses CD15 to gate on neutrophils, and recently uses CD64 to gate on monocytes.
Aims
In this study, we investigate utility of CD14 and CD64 in PNH study of monocyte by compare with CD45 gating in PNH study of all cytopenia cases including underlying hematologic malignancy.
Methods
Total 102 PNH study cases were recruited from July 2017 to February 2018 at Gachon University Gil Medical Center in Korea. The PNH study was done on EDTA blood specimens. Two tubes of whole blood were stained for white blood cells with two combinations consisting of FLAER and CD45 with CD15, CD24 and CD64, CD14 to gate on granulocyte and monocyte, respectively. The sample was analyzed on Cytomics FC500 cytometer. Monocyte proportions were estimated in two ways, one is gating monocyte region in CD45 plus light scatter plot and the other is gating monocyte region in CD64 plus CD14 scatter plot. Reference monocyte proportions were measured by ADVIA 2120i using samples collected together with PNH study sample. Statistical analysis of result with Bland-Altman plot was done with medcalc 15.2.
Results
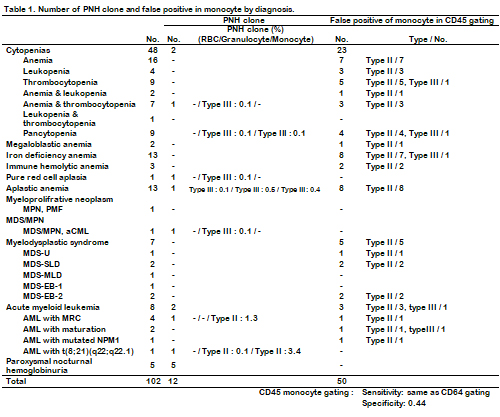
Prominent PNH clone of RBCs, granulocytes and monocytes were identified in 5 cases and minor PNH clones population were detected in 7 cases. 36 cases were undergoing bone marrow biopsy and 17 cases were diagnosed as hematologic malignancy including myelodysplastic syndrome and acute leukemia. Minor PNH clones are identified in 3 hematologic malignancy cases. Aplastic anemia diagnosed in 13 cases, minor PNH clone was detected in one case. CD45 monocyte gating method showed 50 false positive cases. False negative case was not found in this study (Table 1). Compare with CD64 monocyte gating result, specificity of CD45 gating is 0.44 and sensitivity is same as CD64 gating. Difference between reference and CD45 gated monocyte proportion was 105.57(%) with 35.48 standard deviation(SD). Difference between reference and CD14 plus CD64 gated monocyte proportion was 15.87(%) with 36.11 SD.
Conclusion
Flow cytometry allow to detect GPI-deficient PNH phenotype highly sensitive. Adding appropriate monocyte specific lineage markers, such as CD14 and CD64, for gating monocyte result in reduce difference of monocyte proportion between reference method and increases accuracy of monocyte gating. Specificity of detecting PNH clones also increased compare. All 50 false positive samples show single normal monocyte population when tested with CD14 plus CD64 monocyte gating method. Minor PNH population was also detected in CD45 gating method when detected by CD14 plus CD64 gating method. Percentage of type in each minor PNH clone was not much different by gating method. However, Case with prominent PNH clone, proportion of Type III monocytes were less in CD14 plus CD64 gating than CD45 gating, and show more clear population. CD14 and CD64 are useful in PNH study for monocytes detect and identify a small amount of PNH clone, thus these two markers are worthy to include in routine PNH study.
Session topic: 11. Bone marrow failure syndromes incl. PNH – Biology & Translational Research
Keyword(s): flow cytometry, monocyte, PNH
Abstract: PB1821
Type: Publication Only
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare hematopoietic stem cell disorder acquired by somatic mutation of phosphatidylinositol glycan–class A (PIG-A) gene. PIG-A mutation leads partial or absolute defect in expression of GPI-anchored cell surface structure, and this result in episodes of intravascular hemolysis that are typical of the disease. Flow cytometric techniques with utilizing the FLAER enable to sensitive identify very small numbers of GPI-deficient cells. Flow cytometric PNH testing uses CD15 to gate on neutrophils, and recently uses CD64 to gate on monocytes.
Aims
In this study, we investigate utility of CD14 and CD64 in PNH study of monocyte by compare with CD45 gating in PNH study of all cytopenia cases including underlying hematologic malignancy.
Methods
Total 102 PNH study cases were recruited from July 2017 to February 2018 at Gachon University Gil Medical Center in Korea. The PNH study was done on EDTA blood specimens. Two tubes of whole blood were stained for white blood cells with two combinations consisting of FLAER and CD45 with CD15, CD24 and CD64, CD14 to gate on granulocyte and monocyte, respectively. The sample was analyzed on Cytomics FC500 cytometer. Monocyte proportions were estimated in two ways, one is gating monocyte region in CD45 plus light scatter plot and the other is gating monocyte region in CD64 plus CD14 scatter plot. Reference monocyte proportions were measured by ADVIA 2120i using samples collected together with PNH study sample. Statistical analysis of result with Bland-Altman plot was done with medcalc 15.2.
Results
Prominent PNH clone of RBCs, granulocytes and monocytes were identified in 5 cases and minor PNH clones population were detected in 7 cases. 36 cases were undergoing bone marrow biopsy and 17 cases were diagnosed as hematologic malignancy including myelodysplastic syndrome and acute leukemia. Minor PNH clones are identified in 3 hematologic malignancy cases. Aplastic anemia diagnosed in 13 cases, minor PNH clone was detected in one case. CD45 monocyte gating method showed 50 false positive cases. False negative case was not found in this study (Table 1). Compare with CD64 monocyte gating result, specificity of CD45 gating is 0.44 and sensitivity is same as CD64 gating. Difference between reference and CD45 gated monocyte proportion was 105.57(%) with 35.48 standard deviation(SD). Difference between reference and CD14 plus CD64 gated monocyte proportion was 15.87(%) with 36.11 SD.

Conclusion
Flow cytometry allow to detect GPI-deficient PNH phenotype highly sensitive. Adding appropriate monocyte specific lineage markers, such as CD14 and CD64, for gating monocyte result in reduce difference of monocyte proportion between reference method and increases accuracy of monocyte gating. Specificity of detecting PNH clones also increased compare. All 50 false positive samples show single normal monocyte population when tested with CD14 plus CD64 monocyte gating method. Minor PNH population was also detected in CD45 gating method when detected by CD14 plus CD64 gating method. Percentage of type in each minor PNH clone was not much different by gating method. However, Case with prominent PNH clone, proportion of Type III monocytes were less in CD14 plus CD64 gating than CD45 gating, and show more clear population. CD14 and CD64 are useful in PNH study for monocytes detect and identify a small amount of PNH clone, thus these two markers are worthy to include in routine PNH study.
Session topic: 11. Bone marrow failure syndromes incl. PNH – Biology & Translational Research
Keyword(s): flow cytometry, monocyte, PNH


