
Contributions
Abstract: PB2102
Type: Publication Only
Background
The advent of targeted therapy has increased the repertoire of therapeutic options. In particular the methyl transferase inhibitor 5 Azacytidine, that targets epigenetic changes in MDS and AML.The myelodysplastic syndromes (MDS) are an acquired form of clonal stem cell disorders that manifest heterogeneously but are unified clinically by progressive bone marrow failure, susceptibility to life threatening infections, and a risk of transforming to leukemia.We report a single centre experience of using Azacitidine in the licensed NICE indications, comparing with the published data.
Aims
Aim of our study was to assess azacitidine response rates in different cytogenetic risk groups and varying performance status.
Methods
We retrospectively reviewed patients with MDS and AML who received Azacitidine at the Heart of England NHS Foundation Trust from Apr 2012- January 2018. Patient’s demographic data, disease characteristics, treatment, outcome and follow up data were obtained and all patients were included irrespective of the number of the cycles.
Results
57 patients were included in the analysis. Median age of treatment was 73.9 years. Median number of cycles was 6 (range from 1 to 43). There were 68% of MDS patients, 22.8% of AML and 8.8% of CMML2 patients in the cohort . Varying degree of performance stage was noted including ECOG 0,1 and 2 at 40.4%, 43.9% and 7.7% respectively. 42.1% had high risk cytogenetics depending upon the IPSS-R risk based classification. 19.3% patients had complex karyotype (3/>3). Median Hb was 9g/dl, Neutrophil count was 0.7x109/l and platelet count was 41x109/l in this population. Median bone marrow blast percentage was 10%.
Responding patients showed reduced transfusion requirement. 10, 26 and 25 patient had not had any transfusions in first second and third quarters correspondently. Causes of death were mainly disease progression and sepsis. 42.2% died due to disease progression and 35.1% died due to sepsis.
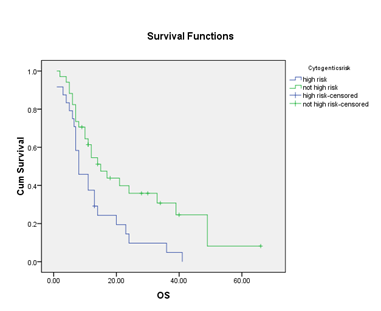
Overall, median progression free survival (PFS) was 19 monts while median overall survival (OS) was 12 months. Overall survival was not affected by age, Bone marrow median blast percentage and median neutrophil count, however, survival was affected by presenting peripheral blast percentage, IPSS-R, Haemoglobin and median platelet count that seemed to be statistically significant (P<0.05). There is no statistically significant PFS or OS for patients who had varying performance stage (p=0.78 and p0.65 respectively). Nevertheless, there is significance difference noted in the group of who had high risk cytogenetic versus other cytogenetic risks with PFS of 8 months and 15 months respectively (p=0.017), OS of 15 and 29 months respectively (p=0.09).
Conclusion
Azacidine therapy has benefited for the patients who had advanced age AML CMML and MDS irrespective of their performance stage at ECOG 0-2 and patient who has non high risk cytogenetic based on IPSS-R risk categorization. However, the group of patients with high risk cytogenetic and poor performance considered historically very poor survival had considerable PFS and OS benefitting treatment rather than best supportive care.
Session topic: 10. Myelodysplastic syndromes – Clinical
Keyword(s): Azacitidine, Cytogenetic abnormalities, MDS
Abstract: PB2102
Type: Publication Only
Background
The advent of targeted therapy has increased the repertoire of therapeutic options. In particular the methyl transferase inhibitor 5 Azacytidine, that targets epigenetic changes in MDS and AML.The myelodysplastic syndromes (MDS) are an acquired form of clonal stem cell disorders that manifest heterogeneously but are unified clinically by progressive bone marrow failure, susceptibility to life threatening infections, and a risk of transforming to leukemia.We report a single centre experience of using Azacitidine in the licensed NICE indications, comparing with the published data.
Aims
Aim of our study was to assess azacitidine response rates in different cytogenetic risk groups and varying performance status.
Methods
We retrospectively reviewed patients with MDS and AML who received Azacitidine at the Heart of England NHS Foundation Trust from Apr 2012- January 2018. Patient’s demographic data, disease characteristics, treatment, outcome and follow up data were obtained and all patients were included irrespective of the number of the cycles.
Results
57 patients were included in the analysis. Median age of treatment was 73.9 years. Median number of cycles was 6 (range from 1 to 43). There were 68% of MDS patients, 22.8% of AML and 8.8% of CMML2 patients in the cohort . Varying degree of performance stage was noted including ECOG 0,1 and 2 at 40.4%, 43.9% and 7.7% respectively. 42.1% had high risk cytogenetics depending upon the IPSS-R risk based classification. 19.3% patients had complex karyotype (3/>3). Median Hb was 9g/dl, Neutrophil count was 0.7x109/l and platelet count was 41x109/l in this population. Median bone marrow blast percentage was 10%.
Responding patients showed reduced transfusion requirement. 10, 26 and 25 patient had not had any transfusions in first second and third quarters correspondently. Causes of death were mainly disease progression and sepsis. 42.2% died due to disease progression and 35.1% died due to sepsis.
Overall, median progression free survival (PFS) was 19 monts while median overall survival (OS) was 12 months. Overall survival was not affected by age, Bone marrow median blast percentage and median neutrophil count, however, survival was affected by presenting peripheral blast percentage, IPSS-R, Haemoglobin and median platelet count that seemed to be statistically significant (P<0.05). There is no statistically significant PFS or OS for patients who had varying performance stage (p=0.78 and p0.65 respectively). Nevertheless, there is significance difference noted in the group of who had high risk cytogenetic versus other cytogenetic risks with PFS of 8 months and 15 months respectively (p=0.017), OS of 15 and 29 months respectively (p=0.09).

Conclusion
Azacidine therapy has benefited for the patients who had advanced age AML CMML and MDS irrespective of their performance stage at ECOG 0-2 and patient who has non high risk cytogenetic based on IPSS-R risk categorization. However, the group of patients with high risk cytogenetic and poor performance considered historically very poor survival had considerable PFS and OS benefitting treatment rather than best supportive care.
Session topic: 10. Myelodysplastic syndromes – Clinical
Keyword(s): Azacitidine, Cytogenetic abnormalities, MDS


