
Contributions
Abstract: PB1921
Type: Publication Only
Background
Several tyrosine kinase inhibitors (TKIs) are available for treatment of patients with chronic myeloid leukaemia in chronic phase (CML-CP).
Aims
We analysed different TKI modalities used as therapy for CML-CP in a long-term analysis
Methods
In a retrospective cohort analysis, we included data from patients with CML-CP treated in clinical practice with TKI modalities at Spanish Registry of CML (RELMC) (17 hospitals around all the country) between 2000 to 2014. The main aim of the study was to describe the sequence of TKI treatment, in real life practice and the rate of last deep molecular response (DMR) (MR4, MR4.5 or undetectable transcript) in each scheme
Results
Our analysis included 862 patients with CML in first chronic phase treated with TKIs in first line or after Interferon alpha. Demographics 517 M, 345 F, median age: 52 y (14-94). Sokal Index distribution (low 49% Inter 38% High 13%), EURO Index distribution (low 50 % Inter 45% High 5 %), EUTOS Index distribution (low 93% High 7%), LT-EUTOS Index distribution (low 68% Inter 25% High 7%).
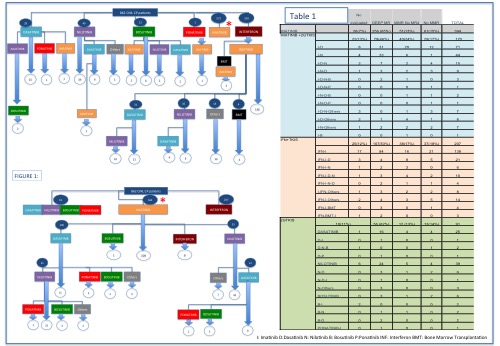
Schemes of treatment: Table 1 summarizes all the schemes used and the last molecular response. Patients were divided in 4 groups depending of TKIs treatment. Group 1: Only treated wit Imatinib 394(45,7%) Group 2: Imatinib and then 2ºGTKIs due to intolerance or failure 170 p (19,7 %) (12 schemes of sequential TKIs treatment) Group 3 2ºGTKs in first line 91 p (13 sequential schemes) (10,5%) Group 4 Interferon alpha and then ITKs: 207 (24 %)(9 sequential schemes). Figure 1 summarizes evolution of different treatments around 14 years.
Last deep molecular response: With a median of follow up of 82 months (1-351 months) from diagnosis, 77 months (1-311 months) from first treatment and 70 months (1-191 months) from first TKI treatment. The rates of deep molecular response for each group were (G1: DMR 65% MMR 13% No MMR 15%, G2: DMR 46% MMR 24% No MMR 17% G3: DMR 62% MMR 13% No MMR 12 % G4: DMR 53% MMR 17% No MMR 18%).
Long-term survival (PFS or OS): We did not find statistical differences between groups of treatment, either from diagnosis, first treatment or first TKI. Reaching a deep response guarantees better outcomes.
Predicting variables: SOKAL, EUTOS, EURO and LT-EUTOs indexes continue to be useful in predicting long-term outcome.
Conclusion
In the setting of a multicentric, hospital-based CML registry, treatment with TKIs is very variable, resulting in a great number of sequential combinations of TKI. The rate of deep molecular response is roughly 60% in patients treated with imatinib and not needing change of TKI, and in those treated upfront with 2ºGTKI, but appears lower in patients treated with imatinib upfront, but who need to switch to 2ºGTKIs. Survival outcomes were not different, although it is worth to point out the shorter follow-up of patients treated upfront with 2ºGTKIs.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, imatinib, Kinase Inhibitor
Abstract: PB1921
Type: Publication Only
Background
Several tyrosine kinase inhibitors (TKIs) are available for treatment of patients with chronic myeloid leukaemia in chronic phase (CML-CP).
Aims
We analysed different TKI modalities used as therapy for CML-CP in a long-term analysis
Methods
In a retrospective cohort analysis, we included data from patients with CML-CP treated in clinical practice with TKI modalities at Spanish Registry of CML (RELMC) (17 hospitals around all the country) between 2000 to 2014. The main aim of the study was to describe the sequence of TKI treatment, in real life practice and the rate of last deep molecular response (DMR) (MR4, MR4.5 or undetectable transcript) in each scheme
Results
Our analysis included 862 patients with CML in first chronic phase treated with TKIs in first line or after Interferon alpha. Demographics 517 M, 345 F, median age: 52 y (14-94). Sokal Index distribution (low 49% Inter 38% High 13%), EURO Index distribution (low 50 % Inter 45% High 5 %), EUTOS Index distribution (low 93% High 7%), LT-EUTOS Index distribution (low 68% Inter 25% High 7%).
Schemes of treatment: Table 1 summarizes all the schemes used and the last molecular response. Patients were divided in 4 groups depending of TKIs treatment. Group 1: Only treated wit Imatinib 394(45,7%) Group 2: Imatinib and then 2ºGTKIs due to intolerance or failure 170 p (19,7 %) (12 schemes of sequential TKIs treatment) Group 3 2ºGTKs in first line 91 p (13 sequential schemes) (10,5%) Group 4 Interferon alpha and then ITKs: 207 (24 %)(9 sequential schemes). Figure 1 summarizes evolution of different treatments around 14 years.
Last deep molecular response: With a median of follow up of 82 months (1-351 months) from diagnosis, 77 months (1-311 months) from first treatment and 70 months (1-191 months) from first TKI treatment. The rates of deep molecular response for each group were (G1: DMR 65% MMR 13% No MMR 15%, G2: DMR 46% MMR 24% No MMR 17% G3: DMR 62% MMR 13% No MMR 12 % G4: DMR 53% MMR 17% No MMR 18%).
Long-term survival (PFS or OS): We did not find statistical differences between groups of treatment, either from diagnosis, first treatment or first TKI. Reaching a deep response guarantees better outcomes.
Predicting variables: SOKAL, EUTOS, EURO and LT-EUTOs indexes continue to be useful in predicting long-term outcome.

Conclusion
In the setting of a multicentric, hospital-based CML registry, treatment with TKIs is very variable, resulting in a great number of sequential combinations of TKI. The rate of deep molecular response is roughly 60% in patients treated with imatinib and not needing change of TKI, and in those treated upfront with 2ºGTKI, but appears lower in patients treated with imatinib upfront, but who need to switch to 2ºGTKIs. Survival outcomes were not different, although it is worth to point out the shorter follow-up of patients treated upfront with 2ºGTKIs.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, imatinib, Kinase Inhibitor


