
Contributions
Abstract: PB1940
Type: Publication Only
Background
The first reports on peripheral arterial occlusive diseases (PAOD) in Chronic Myeloid Leukemia (CML) patients (pts) treated by nilotinib appeared in 2011. Some experimental data showed direct proatherogenic actions of nilotinib (inhibition of DDR1 receptor, possible participation in pathogenic blockage of KIT and PDGFR kinases), possibility to create angiospasm. However detailed mechanisms of cardiovascular events (CVEs) emergence after exposure to nilotinib are not described.
Aims
Evaluate frequency and risk factors of CVEs emergence in CML pts treated by nilotinib.
Methods
119 CML pts treated by nilotinib and monitored in the National Research Center for Hematology of Russia were included in the study 2007 to 2017. Male:female ratio was 45:74. Median age at the start of nilotinib treatment was 43 years (range 23 – 60). 67 (56.3%) pts were transferred to nilotinib due resistance to imatinib, 26 (21.8%) – due to imatinib toxicity, 8 (6.7%) – due to suboptimal response to imatinib, other – 9 (7.6%); 12 pts (10%) were treated by nilotinib as first line therapy, 98 pts (82.3%) were treated by nilotinib as second line therapy after imatinib. 74 pts received 800 mg/day dose of nilotinib, 42 pts – 600 mg/day, 3 – 400 mg/day. We evaluated risk factors (RF) of CVEs, including age, smoking, arterial hypertension presence, overweight, family history of early cardiovascular diseases, type 2 diabetes, glucose level, Lipemic index (total cholesterol and fractions, triglycerides). SCORE scale was used to evaluate RF of death due to CVEs.
Results
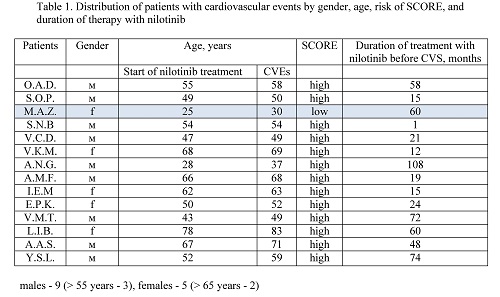
Before nilotinib treatment the following RF of CVEs were found: males > 55 years – 8 (17.7%), females > 65 years – 7 (9.4%), smoking – 28 (23.5%), increased level of total cholesterol –39 (32.7%), overweight – 34 (28.6%), hyperglycemia – 12 (10%), family history of early CVEs – 6 (5%), arterial hypertension – 29 (39.5%), type 2 diabetes – 8 (6.7%). SCORE distribution was as follows: low risk – 64, moderate – 18, high – 8, very high – 11, no data – 18. In nilotinib treated pts at 14 (11.7%) (9 males (20%) and 5 females (6.8%)) the following CVEs were observed: Acute Cerebrovascular Event (ACE) – 7 (5.8%), PAOD – 2 (1.7%), first time observed angina pectoris – 5 (4.2%). 13 of 14 of the pts mentioned above (92.8%) had a high risk according to SCORE scale before nilotinib treatment. Transfer to other tyrosine kinase inhibitor (TKI) – 18 (15%): dasatinib - 12, bosutinib – 2, ponatinib – 1, imatinib – 1, PF114 – 2; observation without treatment – 29 (24%). 12 pts (10%) died, of which 8 (6.7%) – due to disease progression, 4 (3.4%) – due to CVEs, 2 (1.7%) – due to nilotinib treatment. 60 pts (50.4%) continue nilotinib treatment with observation median of 52.6 months (1 – 135.8).
Conclusion
Combination of obtained data allows to propose that nilotinib can be an additional factor of accelerated development of atherosclerosis leading to various CVEs. It cannot be excluded that CVEs emergence can be linked to nilotinib dose as most pts with CVEs received maximum possible dose – 800 mg/day, apart from 3 pts taking lesser dose (2 – 600 mg/day, 1 – 400 mg/day). All pts with observed CVEs, excluding one, had a high risk of CVEs in accordance with SCORE scale. Taking into account possible correlation of CVEs frequency in nilotinib treated pts with high risk in accordance with SCORE scale it is recommended to estimate RF during screening to prevent CVEs emergence. At increased risk of CVEs emergence it is recommended to correct RF, which can be modified, decrease nilotinib dose or transfer to other TKI.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Atherosclerosis, Chronic myeloid leukemia, Nilotinib, Tyrosine kinase inhibitor
Abstract: PB1940
Type: Publication Only
Background
The first reports on peripheral arterial occlusive diseases (PAOD) in Chronic Myeloid Leukemia (CML) patients (pts) treated by nilotinib appeared in 2011. Some experimental data showed direct proatherogenic actions of nilotinib (inhibition of DDR1 receptor, possible participation in pathogenic blockage of KIT and PDGFR kinases), possibility to create angiospasm. However detailed mechanisms of cardiovascular events (CVEs) emergence after exposure to nilotinib are not described.
Aims
Evaluate frequency and risk factors of CVEs emergence in CML pts treated by nilotinib.
Methods
119 CML pts treated by nilotinib and monitored in the National Research Center for Hematology of Russia were included in the study 2007 to 2017. Male:female ratio was 45:74. Median age at the start of nilotinib treatment was 43 years (range 23 – 60). 67 (56.3%) pts were transferred to nilotinib due resistance to imatinib, 26 (21.8%) – due to imatinib toxicity, 8 (6.7%) – due to suboptimal response to imatinib, other – 9 (7.6%); 12 pts (10%) were treated by nilotinib as first line therapy, 98 pts (82.3%) were treated by nilotinib as second line therapy after imatinib. 74 pts received 800 mg/day dose of nilotinib, 42 pts – 600 mg/day, 3 – 400 mg/day. We evaluated risk factors (RF) of CVEs, including age, smoking, arterial hypertension presence, overweight, family history of early cardiovascular diseases, type 2 diabetes, glucose level, Lipemic index (total cholesterol and fractions, triglycerides). SCORE scale was used to evaluate RF of death due to CVEs.
Results
Before nilotinib treatment the following RF of CVEs were found: males > 55 years – 8 (17.7%), females > 65 years – 7 (9.4%), smoking – 28 (23.5%), increased level of total cholesterol –39 (32.7%), overweight – 34 (28.6%), hyperglycemia – 12 (10%), family history of early CVEs – 6 (5%), arterial hypertension – 29 (39.5%), type 2 diabetes – 8 (6.7%). SCORE distribution was as follows: low risk – 64, moderate – 18, high – 8, very high – 11, no data – 18. In nilotinib treated pts at 14 (11.7%) (9 males (20%) and 5 females (6.8%)) the following CVEs were observed: Acute Cerebrovascular Event (ACE) – 7 (5.8%), PAOD – 2 (1.7%), first time observed angina pectoris – 5 (4.2%). 13 of 14 of the pts mentioned above (92.8%) had a high risk according to SCORE scale before nilotinib treatment. Transfer to other tyrosine kinase inhibitor (TKI) – 18 (15%): dasatinib - 12, bosutinib – 2, ponatinib – 1, imatinib – 1, PF114 – 2; observation without treatment – 29 (24%). 12 pts (10%) died, of which 8 (6.7%) – due to disease progression, 4 (3.4%) – due to CVEs, 2 (1.7%) – due to nilotinib treatment. 60 pts (50.4%) continue nilotinib treatment with observation median of 52.6 months (1 – 135.8).

Conclusion
Combination of obtained data allows to propose that nilotinib can be an additional factor of accelerated development of atherosclerosis leading to various CVEs. It cannot be excluded that CVEs emergence can be linked to nilotinib dose as most pts with CVEs received maximum possible dose – 800 mg/day, apart from 3 pts taking lesser dose (2 – 600 mg/day, 1 – 400 mg/day). All pts with observed CVEs, excluding one, had a high risk of CVEs in accordance with SCORE scale. Taking into account possible correlation of CVEs frequency in nilotinib treated pts with high risk in accordance with SCORE scale it is recommended to estimate RF during screening to prevent CVEs emergence. At increased risk of CVEs emergence it is recommended to correct RF, which can be modified, decrease nilotinib dose or transfer to other TKI.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Atherosclerosis, Chronic myeloid leukemia, Nilotinib, Tyrosine kinase inhibitor


