
Contributions
Abstract: PB1932
Type: Publication Only
Background
Nilotinib (NILO) administration in CML patients (pts) is known to be associated with impairment of lipid metabolism, which is one of the possible risk factors leading to cardiovascular (CV) adverse events (AE) of NILO. On the contrary, these abnormalities were not observed during imatinib (IMA) treatment. A routine protocol for lipid and CV risk assessment at the start and during both TKIs administration is still not established.
Aims
1) To analyse the serum lipids levels and CV risk in de novo CML pts treated with NILO and IMA at baseline and during the therapy. 2) To identify CML pts who meet the criteria for a hypolipidemic treatment according ESC/EAS guidelines at diagnosis and during the TKI therapy.
Methods
Consecutive pts diagnosed with CML between 6/2014 - 7/2017 treated with NILO or IMA in the 1st line were included in this prospective study. The selection of TKI was based on treating physician’s decision. Patients received clinical and laboratory diagnostic workup and CV risk assessment according to ESC/EAS at the start of TKI therapy and then every 3 months for two years. Changes in serum lipids levels were analysed only in pts without hypolipidemic therapy at the study visit.
Results
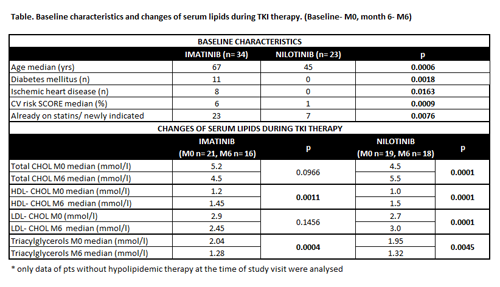
Thirty- four pts treated with IMA and 23 with NILO were included in this trial (median follow-up: IMA = 12m, NILO = 9m). Pts in NILO group were significantly younger compared to IMA (median age – 45 vs. 67 yrs; p = 0.0006) and had significantly less comorbidities (diabetes mellitus 0% vs. 32%, p = 0.0018; ischemic heart disease – 0% vs. 24%; p = 0.0163). The baseline CV risk SCORE value of NILO pts was also significantly lower (SCORE – median - 6% vs. 1%; p = 0.009). Moreover, there were significantly more pts already or newly indicated for statins at the start of IMA [IMA 68% (7/34 pts on statin, 16/34 pts newly indicated) vs. NILO 30% (3/23 pts on statin, 4/23 newly indicated); p = 0.0076]. These differences between groups reflect common selection of TKIs by treating physicians based on their known AEs. Baseline levels of total- and LDL- cholesterol (CHOL) between groups were not different, but this could be affected by different percentage of patients already on statins in both groups. During the first 6 months of treatment the level of total- and LDL- CHOL in NILO pts significantly increased. Total- and LDL- CHOL did not change in IMA pts. HDL cholesterol significantly increased in both groups (Table). During the first 6 months 2/34 (6%) IMA pts and 3/23 (13%) NILO pts previously classified at low CV risk moved to high or very high CV risk category (p = ns). No new CV event occurred in both groups. The number of pts additionally indicated for statin therapy during the first 6 months of TKI treatment was similar in both groups [IMA vs. NILO – 2/34 (5,9%) vs 2/23 (8,7%); p = ns].
Conclusion
Our prospective real-life study clearly showed the routine selection of TKI by treating physician in de novo CML based on comorbidities and age. Importantly, there is high percentage of patients with de novo CML in central European population who fulfil criteria for statins already at the start of TKI therapy (53% in our study). Even though NILO increases the total/LDL- CHOL, the increase of CV risk and number of pts newly indicated for statin therapy does not differ from the IMA group, at least not during the first 6 months of therapy. However, this may be influenced by mentioned routine selection of TKI in CML patients and also longer follow-up is needed.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Adverse reaction, Chronic myeloid leukemia, Tyrosine kinase inhibitor
Abstract: PB1932
Type: Publication Only
Background
Nilotinib (NILO) administration in CML patients (pts) is known to be associated with impairment of lipid metabolism, which is one of the possible risk factors leading to cardiovascular (CV) adverse events (AE) of NILO. On the contrary, these abnormalities were not observed during imatinib (IMA) treatment. A routine protocol for lipid and CV risk assessment at the start and during both TKIs administration is still not established.
Aims
1) To analyse the serum lipids levels and CV risk in de novo CML pts treated with NILO and IMA at baseline and during the therapy. 2) To identify CML pts who meet the criteria for a hypolipidemic treatment according ESC/EAS guidelines at diagnosis and during the TKI therapy.
Methods
Consecutive pts diagnosed with CML between 6/2014 - 7/2017 treated with NILO or IMA in the 1st line were included in this prospective study. The selection of TKI was based on treating physician’s decision. Patients received clinical and laboratory diagnostic workup and CV risk assessment according to ESC/EAS at the start of TKI therapy and then every 3 months for two years. Changes in serum lipids levels were analysed only in pts without hypolipidemic therapy at the study visit.
Results
Thirty- four pts treated with IMA and 23 with NILO were included in this trial (median follow-up: IMA = 12m, NILO = 9m). Pts in NILO group were significantly younger compared to IMA (median age – 45 vs. 67 yrs; p = 0.0006) and had significantly less comorbidities (diabetes mellitus 0% vs. 32%, p = 0.0018; ischemic heart disease – 0% vs. 24%; p = 0.0163). The baseline CV risk SCORE value of NILO pts was also significantly lower (SCORE – median - 6% vs. 1%; p = 0.009). Moreover, there were significantly more pts already or newly indicated for statins at the start of IMA [IMA 68% (7/34 pts on statin, 16/34 pts newly indicated) vs. NILO 30% (3/23 pts on statin, 4/23 newly indicated); p = 0.0076]. These differences between groups reflect common selection of TKIs by treating physicians based on their known AEs. Baseline levels of total- and LDL- cholesterol (CHOL) between groups were not different, but this could be affected by different percentage of patients already on statins in both groups. During the first 6 months of treatment the level of total- and LDL- CHOL in NILO pts significantly increased. Total- and LDL- CHOL did not change in IMA pts. HDL cholesterol significantly increased in both groups (Table). During the first 6 months 2/34 (6%) IMA pts and 3/23 (13%) NILO pts previously classified at low CV risk moved to high or very high CV risk category (p = ns). No new CV event occurred in both groups. The number of pts additionally indicated for statin therapy during the first 6 months of TKI treatment was similar in both groups [IMA vs. NILO – 2/34 (5,9%) vs 2/23 (8,7%); p = ns].

Conclusion
Our prospective real-life study clearly showed the routine selection of TKI by treating physician in de novo CML based on comorbidities and age. Importantly, there is high percentage of patients with de novo CML in central European population who fulfil criteria for statins already at the start of TKI therapy (53% in our study). Even though NILO increases the total/LDL- CHOL, the increase of CV risk and number of pts newly indicated for statin therapy does not differ from the IMA group, at least not during the first 6 months of therapy. However, this may be influenced by mentioned routine selection of TKI in CML patients and also longer follow-up is needed.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Adverse reaction, Chronic myeloid leukemia, Tyrosine kinase inhibitor


