
Contributions
Abstract: PB1927
Type: Publication Only
Background
Currently, there lacks available real-world clinical efficacy data on different strategies, such as comparing the efficacy of frontline nilotinib with imatinib and the efficacy of second-line nilotinib in Pts resistant or intolerant to imatinib. Meanwhile, when to apply the second generation of TKIs instead of the first generation of TKIs as well as their clinical efficacy hasn’t been defined.
Aims
The present study was aimed to explore these issues and optimize clinical therapy through evaluating molecular response at specific milestones according to ELN guidelines.
Methods
We have enrolled 319 Pts from our hospital from January 2000 to December 2017 who were newly diagnosed with CML. 29 Pts received frontline nilotinib and the other 290 received imatinib .The study was descriptively analyzed and evaluated the efficacy of imatinib and nilotinib.
Results
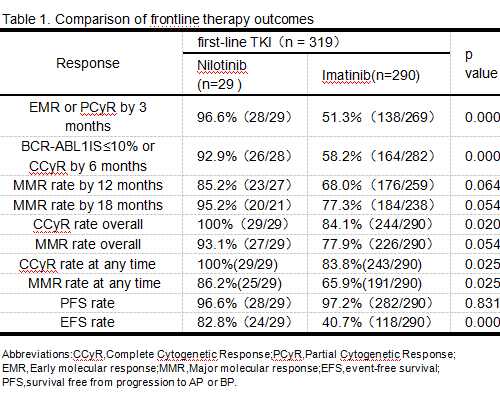
At data collection, CML-CP was confirmed in 309 of 319 Pts who were newly diagnosed, and CML-AP was confirmed in 10 of 319 the Pts. 210 (65.8%) Pts were male. Median follow-up was 45 (range 3 to 199) months and median age was 39 (range 2 to 76) years. The molecular responses of frontline therapy at ELN2013 milestones during the observation period are shown in Table 1. During the observation period, 84/290(29%) Pts with frontline imatinib switched to nilotinib; 32/84 switched early (within 12 months) and 52/84 switched later.Rates of CCyR at any time for Pts who switched early and later were 90.6%(29/32) and 88.5%(46/22) (P=0.756).Rates of MMR at any time for Pts who switched early and later were 84.4%(27/32) and 76.9%(40/52) (P=0.409). The documented main reason for first switch was treatment failure[(50/84(59.5%) Pts, medium time was 19.5 months] while 12/84 Pts (median time was 11 months) were switched for “warning”, 15 (17.9%) Pts were switch for intolerance(median time was 21 months), 6/84 (7.1%) Pts were switched for willing to withdrawal (medium time was 25.5 months) and one for unexplained eosinophilia(time was 35 months). Rates of CCyR and MMR at any time for Pts who switched TKI following a prior "warning " response were 83.3%(10/12) and 75%(9/12). Rates of CCyR and MMR at any time for Pts who switched TKI following a prior "failure " response were 86%(43/50) and 72%(36/50). 206/290 Pts remained on frontline imatinib with no observed switch. In the 54/206 Pts following “warning” response, 22/54 had a “failure” response at one or more ELN2013 milestones. In the 133/206 Pts following “optimal” response, 4/133 had a “failure” response and 1/133 had a “warning” response. Rates of CCyR and MMR at any time for Pts who remained on frontline imatinib following a prior "warning" response were 87.0%(47/54) and 66.7%(36/54). Rates of CCyR and MMR at any time for Pts who remained on frontline imatinib following a prior "failure" response were 78.9%(15/19) and 63.2%(12/19). Rates of CCyR and MMR at any time in Pts were 93.1%(270/290) and 82.8%(240/290) separately, significantly higher than that in the frontline imatinib group and similar to the rate of CCyR and MMR(100% and 86.2%) in the frontline nilotinib group.
Conclusion
As the first-line drug for newly diagnosed CML Pts, Nilotinib can achieve a deeper molecular response in a shorter time than imatinib. According to ELN’s suggestion, the clinical efficacy of Pts who switch early to nilotinib for resistant or intolerant to imatinib can reach similar efficacy as the first-line nilotinib. Overall, the results support the use of ELN2013 recommendations to guide TKI management.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Imatinib resistance, Tyrosine kinase inhibitor
Abstract: PB1927
Type: Publication Only
Background
Currently, there lacks available real-world clinical efficacy data on different strategies, such as comparing the efficacy of frontline nilotinib with imatinib and the efficacy of second-line nilotinib in Pts resistant or intolerant to imatinib. Meanwhile, when to apply the second generation of TKIs instead of the first generation of TKIs as well as their clinical efficacy hasn’t been defined.
Aims
The present study was aimed to explore these issues and optimize clinical therapy through evaluating molecular response at specific milestones according to ELN guidelines.
Methods
We have enrolled 319 Pts from our hospital from January 2000 to December 2017 who were newly diagnosed with CML. 29 Pts received frontline nilotinib and the other 290 received imatinib .The study was descriptively analyzed and evaluated the efficacy of imatinib and nilotinib.
Results
At data collection, CML-CP was confirmed in 309 of 319 Pts who were newly diagnosed, and CML-AP was confirmed in 10 of 319 the Pts. 210 (65.8%) Pts were male. Median follow-up was 45 (range 3 to 199) months and median age was 39 (range 2 to 76) years. The molecular responses of frontline therapy at ELN2013 milestones during the observation period are shown in Table 1. During the observation period, 84/290(29%) Pts with frontline imatinib switched to nilotinib; 32/84 switched early (within 12 months) and 52/84 switched later.Rates of CCyR at any time for Pts who switched early and later were 90.6%(29/32) and 88.5%(46/22) (P=0.756).Rates of MMR at any time for Pts who switched early and later were 84.4%(27/32) and 76.9%(40/52) (P=0.409). The documented main reason for first switch was treatment failure[(50/84(59.5%) Pts, medium time was 19.5 months] while 12/84 Pts (median time was 11 months) were switched for “warning”, 15 (17.9%) Pts were switch for intolerance(median time was 21 months), 6/84 (7.1%) Pts were switched for willing to withdrawal (medium time was 25.5 months) and one for unexplained eosinophilia(time was 35 months). Rates of CCyR and MMR at any time for Pts who switched TKI following a prior "warning " response were 83.3%(10/12) and 75%(9/12). Rates of CCyR and MMR at any time for Pts who switched TKI following a prior "failure " response were 86%(43/50) and 72%(36/50). 206/290 Pts remained on frontline imatinib with no observed switch. In the 54/206 Pts following “warning” response, 22/54 had a “failure” response at one or more ELN2013 milestones. In the 133/206 Pts following “optimal” response, 4/133 had a “failure” response and 1/133 had a “warning” response. Rates of CCyR and MMR at any time for Pts who remained on frontline imatinib following a prior "warning" response were 87.0%(47/54) and 66.7%(36/54). Rates of CCyR and MMR at any time for Pts who remained on frontline imatinib following a prior "failure" response were 78.9%(15/19) and 63.2%(12/19). Rates of CCyR and MMR at any time in Pts were 93.1%(270/290) and 82.8%(240/290) separately, significantly higher than that in the frontline imatinib group and similar to the rate of CCyR and MMR(100% and 86.2%) in the frontline nilotinib group.

Conclusion
As the first-line drug for newly diagnosed CML Pts, Nilotinib can achieve a deeper molecular response in a shorter time than imatinib. According to ELN’s suggestion, the clinical efficacy of Pts who switch early to nilotinib for resistant or intolerant to imatinib can reach similar efficacy as the first-line nilotinib. Overall, the results support the use of ELN2013 recommendations to guide TKI management.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Imatinib resistance, Tyrosine kinase inhibitor


