
Contributions
Abstract: PB1915
Type: Publication Only
Background
Discontinuation of tyrosine kinase inhibitors (TKI) treatment in patients (pts) with chronic myeloid leukemia (CML) having deep molecular response (DMR) now is moving from clinical trials into routine clinical practice. The loss of major molecular response (MMR) with BCR-ABL>0.1% by IS is generally defined as a molecular relapse and a trigger to restart therapy. The BCR-ABL level at molecular relapse and the impact of the delay in treatment resuming after MMR loss which may happen in routine clinical setting are rarely evaluated.
Aims
To evaluate the restoring of DMR in CML pts after resuming of TKI therapy in accordance with the time of therapy restart.
Methods
We observed 70 pts with CML (68 in chronic phase, 2 in accelerated phase at diagnosis) who stopped TKIs being in stable DMR> 1 year. DMR was considered as at least MR4 (BCR-ABL<0.01%). The follow-up was done by quantitative PCR detection of BCR-ABL level. The data were collected retrospectively and prospectively in 2 central clinics of Moscow (n=66) and St.Petersburg (n=4) during years 2008-2016 outside of clinical trials.
The reasons to stop TKI were toxicity (n=30), pregnancy (n=18) and patients’ decision (n=22). Median (Me) time of observation after TKI cessation was 29 months (range 3-120). The low/intermediate/high Sokal risk group was in 45(64%)/ 14(22%)/ 9(14%) of pts respectively. Imatinib/nilotinib/dasatinib/bosutinib were used before treatment cessation in 45 (64%)/ 15(21%)/ 9(13%)/ 1(2%) of pts. Me time of TKI therapy was 6 years (IQR 4-9). Me time of DMR duration was 1.8 years (IQR 2.8-5). TKI were resumed after MMR loss or by physician’s decision. The delays in treatment restart were related to drug access, patients’ decisions or pregnancy. Event free survival (EFS) was evaluated considering MMR loss, TKI resuming and death as the events. Cumulative incidence (CI) of DMR restoring was evaluated in accordance with the time since MMR loss to TKI restart.
Results
The EFS after TKI discontinuation was 69%, 50% and 39% at 6,12 and 24 months(mo) accordingly. Two patients with DMR died from cardiovascular disease. TKIs were resumed in 38 (54%) pts, no MMR loss was in 6 pts at treatment restart. The MMR loss occurred in 32 (46%) of pts with the Me of BCR-ABL level 0.54% by IS (range 0.11% -13%). Me time from MMR loss detection to TKI resuming was 38 days (range 3-276). Treatment was restarted within <30 days in 14 pts and later than 30 days in 18 pts. MR2 loss (BCR-ABL>1%) without hematologic relapse (HR) was in 16 pts at TKI resuming. No HR occurred in 4 pts with MR2 loss and treatment delay of 111-276 days. A HR was observed in 2 pts who did not restart treatment in 122 and 155 days after MMR loss. The same TKI was reinitiated in 30 pts. TKI change due to previous toxicity or by administrative reasons was in 7 pts and 1 pt accordingly. TKI was also changed in 1 pt without MR2 after 6 mo of treatment restart and a DMR was achieved thereafter.
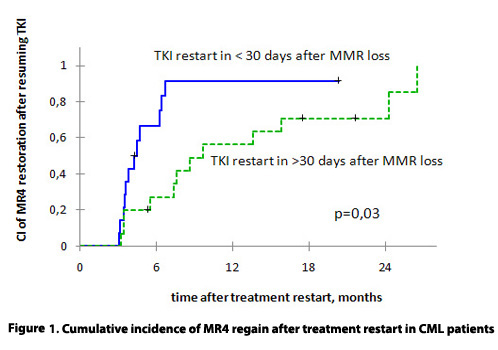
Me time of observation after TKI resuming was 24 mo (range 2-116). CI of DMR regain was 73% and 100% after 12 and 24 months of treatment restart. DMR regain was observed later in pts who resumed TKIs later than 30 days after loss of MMR (figure 1).
Conclusion
A leukemic clone in CML patients may remain sensitive to TKI during prolonged treatment interruptions. However the delays in treatment restart may lead to a HR and a switch to other TKI may be required. CML patients should have regular molecular monitoring during off-treatment period and after TKI resuming.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Molecular relapse, Molecular response
Abstract: PB1915
Type: Publication Only
Background
Discontinuation of tyrosine kinase inhibitors (TKI) treatment in patients (pts) with chronic myeloid leukemia (CML) having deep molecular response (DMR) now is moving from clinical trials into routine clinical practice. The loss of major molecular response (MMR) with BCR-ABL>0.1% by IS is generally defined as a molecular relapse and a trigger to restart therapy. The BCR-ABL level at molecular relapse and the impact of the delay in treatment resuming after MMR loss which may happen in routine clinical setting are rarely evaluated.
Aims
To evaluate the restoring of DMR in CML pts after resuming of TKI therapy in accordance with the time of therapy restart.
Methods
We observed 70 pts with CML (68 in chronic phase, 2 in accelerated phase at diagnosis) who stopped TKIs being in stable DMR> 1 year. DMR was considered as at least MR4 (BCR-ABL<0.01%). The follow-up was done by quantitative PCR detection of BCR-ABL level. The data were collected retrospectively and prospectively in 2 central clinics of Moscow (n=66) and St.Petersburg (n=4) during years 2008-2016 outside of clinical trials.
The reasons to stop TKI were toxicity (n=30), pregnancy (n=18) and patients’ decision (n=22). Median (Me) time of observation after TKI cessation was 29 months (range 3-120). The low/intermediate/high Sokal risk group was in 45(64%)/ 14(22%)/ 9(14%) of pts respectively. Imatinib/nilotinib/dasatinib/bosutinib were used before treatment cessation in 45 (64%)/ 15(21%)/ 9(13%)/ 1(2%) of pts. Me time of TKI therapy was 6 years (IQR 4-9). Me time of DMR duration was 1.8 years (IQR 2.8-5). TKI were resumed after MMR loss or by physician’s decision. The delays in treatment restart were related to drug access, patients’ decisions or pregnancy. Event free survival (EFS) was evaluated considering MMR loss, TKI resuming and death as the events. Cumulative incidence (CI) of DMR restoring was evaluated in accordance with the time since MMR loss to TKI restart.
Results
The EFS after TKI discontinuation was 69%, 50% and 39% at 6,12 and 24 months(mo) accordingly. Two patients with DMR died from cardiovascular disease. TKIs were resumed in 38 (54%) pts, no MMR loss was in 6 pts at treatment restart. The MMR loss occurred in 32 (46%) of pts with the Me of BCR-ABL level 0.54% by IS (range 0.11% -13%). Me time from MMR loss detection to TKI resuming was 38 days (range 3-276). Treatment was restarted within <30 days in 14 pts and later than 30 days in 18 pts. MR2 loss (BCR-ABL>1%) without hematologic relapse (HR) was in 16 pts at TKI resuming. No HR occurred in 4 pts with MR2 loss and treatment delay of 111-276 days. A HR was observed in 2 pts who did not restart treatment in 122 and 155 days after MMR loss. The same TKI was reinitiated in 30 pts. TKI change due to previous toxicity or by administrative reasons was in 7 pts and 1 pt accordingly. TKI was also changed in 1 pt without MR2 after 6 mo of treatment restart and a DMR was achieved thereafter.
Me time of observation after TKI resuming was 24 mo (range 2-116). CI of DMR regain was 73% and 100% after 12 and 24 months of treatment restart. DMR regain was observed later in pts who resumed TKIs later than 30 days after loss of MMR (figure 1).

Conclusion
A leukemic clone in CML patients may remain sensitive to TKI during prolonged treatment interruptions. However the delays in treatment restart may lead to a HR and a switch to other TKI may be required. CML patients should have regular molecular monitoring during off-treatment period and after TKI resuming.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Molecular relapse, Molecular response


