
Contributions
Abstract: PB1913
Type: Publication Only
Background
Imatinib is the most commonly used drug in chronic myeloid leukemia (CML) patients (pts) worldwide. In early 2017 a generic formulation was introduced in Italy and CML pts have switched from branded (i.e. Glivec®, Novartis) to generic imatinib upon requirement of regional health authorities. Since the use of generic drugs represents a novelty in cancer field, some concerns exist about efficacy and safety in comparison to their originators.
Aims
To analyze the outcome of CML pts switched from branded to generic imatinib for changes in adverse event (AE) profile or efficacy.
Methods
We analyzed a cohort of 294 chronic phase CML pts treated in 10 hematological centers with branded imatinib for at least 6 consecutive months before switching to a generic formulation. After switching, RQ-PCR and biochemical exams were performed at least every 3 months and the assessment of AE was continuously performed. Molecular responses were defined according to the ELN2013 recommendations. The severity of AE was assessed according to the CTCAE 4.0 scale.
Results
Median age at diagnosis was 57 years (range 19-87 years). Sokal score was L/I/H in 162 (55%), 93 (32%) and 24 (8%) pts, respectively (15 cases were not evaluable). Median duration of branded imatinib treatment was 7.4 years (range 0.5-16.7 years). Imatinib dose at switch was 400 mg, less than 400 mg and more than 400 mg daily in 71%, 27%, and 2% of pts, respectively. Imatinib dose was not changed at the time of switch.
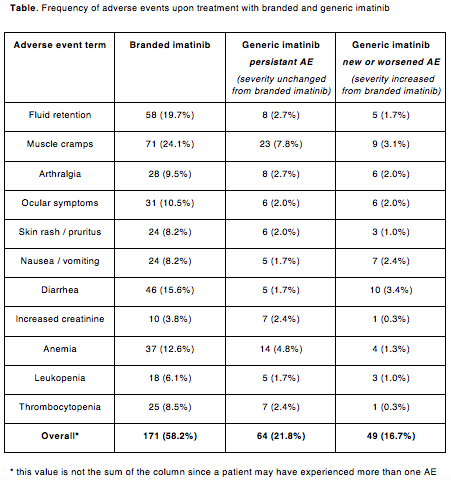
The majority of pts (171/294, 58%) had experienced at least one AE while on branded imatinib, most commonly muscle cramps, fluid retention, diarrhea and anemia (table). Grade 3-4 non hematological AE were uncommon and included infections (n=4), arrhythmia (n=2) ischemic stroke (n=1) and cardiac failure (n=1). Of note, 9 pts had a secondary neoplasm diagnosed while on branded imatinib. At the time of switch molecular responses were as follows: less than MR3 in 25 (8%), MR3 in 75 (26%), MR4 in 87 (30%) and MR4.5 or better in 107 (36%) pts.
At a median follow-up of 7.5 months after switch to generic imatinib (range 0-12.2 months), 49 pts (17%) reported new or worsening AE (table), most commonly nausea, diarrhea and muscle cramps. Grade 3-4 non hematological AE included increase of lipase (n=3), infections (n=2), vomiting (n=1), muscle pain (n=1), and severe allergic reaction at the first intake of generic imatinib (n=1). Twelve pts (4%) interrupted generic imatinib for >30 days and 20 pts (7%) had the dose permanently reduced, most commonly to 200 mg daily. Twenty-two pts (7.5%) discontinued generic imatinib treatment for intolerance (n=9), treatment-free remission attempt (n=8), lack of molecular response (n=3) and death (n=2, both unrelated to CML). Overall, 6 pts (2% of the whole population) switched back to branded imatinib, with improvement in the AE profile, and 4 pts moved to bosutinib (n=3) or nilotinib (n=1). The efficacy of switch was evaluable in 282 pts. Molecular responses remained the same in 229 pts (81%), improved in 37 pts (13%) (from less than MR3 to MR3 or better: n=13; from MR3 to MR4 or better: n=24) and worsened in 16 pts (6%) (from MR3 to less than MR3: n=3; from MR4 or MR4.5 to MR3: n=13).
Conclusion
Switch to generic imatinib for pts who have been receiving branded imatinib for at least 6 months appears to be effective and safe. Molecular responses may continue to improve over time. Some pts experienced new or worsened AE but in our cohort only 3.4% of pts needed to switch back to branded imatinib or move to other TKIs.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Generic drugs, imatinib, Safety
Abstract: PB1913
Type: Publication Only
Background
Imatinib is the most commonly used drug in chronic myeloid leukemia (CML) patients (pts) worldwide. In early 2017 a generic formulation was introduced in Italy and CML pts have switched from branded (i.e. Glivec®, Novartis) to generic imatinib upon requirement of regional health authorities. Since the use of generic drugs represents a novelty in cancer field, some concerns exist about efficacy and safety in comparison to their originators.
Aims
To analyze the outcome of CML pts switched from branded to generic imatinib for changes in adverse event (AE) profile or efficacy.
Methods
We analyzed a cohort of 294 chronic phase CML pts treated in 10 hematological centers with branded imatinib for at least 6 consecutive months before switching to a generic formulation. After switching, RQ-PCR and biochemical exams were performed at least every 3 months and the assessment of AE was continuously performed. Molecular responses were defined according to the ELN2013 recommendations. The severity of AE was assessed according to the CTCAE 4.0 scale.
Results
Median age at diagnosis was 57 years (range 19-87 years). Sokal score was L/I/H in 162 (55%), 93 (32%) and 24 (8%) pts, respectively (15 cases were not evaluable). Median duration of branded imatinib treatment was 7.4 years (range 0.5-16.7 years). Imatinib dose at switch was 400 mg, less than 400 mg and more than 400 mg daily in 71%, 27%, and 2% of pts, respectively. Imatinib dose was not changed at the time of switch.
The majority of pts (171/294, 58%) had experienced at least one AE while on branded imatinib, most commonly muscle cramps, fluid retention, diarrhea and anemia (table). Grade 3-4 non hematological AE were uncommon and included infections (n=4), arrhythmia (n=2) ischemic stroke (n=1) and cardiac failure (n=1). Of note, 9 pts had a secondary neoplasm diagnosed while on branded imatinib. At the time of switch molecular responses were as follows: less than MR3 in 25 (8%), MR3 in 75 (26%), MR4 in 87 (30%) and MR4.5 or better in 107 (36%) pts.
At a median follow-up of 7.5 months after switch to generic imatinib (range 0-12.2 months), 49 pts (17%) reported new or worsening AE (table), most commonly nausea, diarrhea and muscle cramps. Grade 3-4 non hematological AE included increase of lipase (n=3), infections (n=2), vomiting (n=1), muscle pain (n=1), and severe allergic reaction at the first intake of generic imatinib (n=1). Twelve pts (4%) interrupted generic imatinib for >30 days and 20 pts (7%) had the dose permanently reduced, most commonly to 200 mg daily. Twenty-two pts (7.5%) discontinued generic imatinib treatment for intolerance (n=9), treatment-free remission attempt (n=8), lack of molecular response (n=3) and death (n=2, both unrelated to CML). Overall, 6 pts (2% of the whole population) switched back to branded imatinib, with improvement in the AE profile, and 4 pts moved to bosutinib (n=3) or nilotinib (n=1). The efficacy of switch was evaluable in 282 pts. Molecular responses remained the same in 229 pts (81%), improved in 37 pts (13%) (from less than MR3 to MR3 or better: n=13; from MR3 to MR4 or better: n=24) and worsened in 16 pts (6%) (from MR3 to less than MR3: n=3; from MR4 or MR4.5 to MR3: n=13).

Conclusion
Switch to generic imatinib for pts who have been receiving branded imatinib for at least 6 months appears to be effective and safe. Molecular responses may continue to improve over time. Some pts experienced new or worsened AE but in our cohort only 3.4% of pts needed to switch back to branded imatinib or move to other TKIs.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Generic drugs, imatinib, Safety


