
Contributions
Abstract: PB1929
Type: Publication Only
Background
Tyrosine kinase inhibitors (TKIs) are the mainstay of chronic myeloid leukemia (CML) treatment. Median age of CML at diagnosis is 50 years, but a significant proportion of the patients (pts) are diagnosed after age 60. Pts with CML in chronic phase (CML-CP) nowadays live near-normal lifespans, thus, the number of elderly CML pts with comorbidities started to increase.This brings out the issues regarding TKI toxicities, medication adherence and responses to TKI therapy, and we have previously presented the real-life data regarding toxicity and efficacy of imatinib (IM) in our elderly CML cohort [Blood. 2016;128:1905].
Aims
The aim of this study is to update the previously presented data showing the efficacy and safety of IM in the elderly population (pts≥60 years; Group A) with CML and to compare these data with younger patients (pts<60 years; Group B).
Methods
Patient demographics, dose and duration of IM therapy, disease risk scores, cytogenetic and molecular responses, comorbidities, adverse events (AEs), follow-up durations and outcomes were evaluated from pts' files, retrospectively. The Charlson Comorbidity Index (CCI) of each patient was calculated as stated before [Haematologica. 2011;96(10):1457-61].
Results
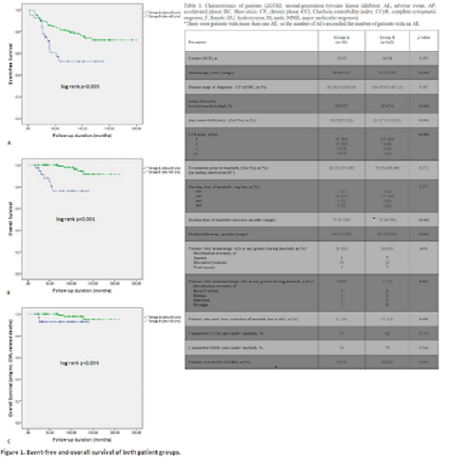
Our cohort consisted of 181 pts with a median age of 46 years (range, 18-83 years). Group A consisted of 39 pts, and there were 142 pts in Group B (Table 1). The two groups were balanced regarding gender, disease stage, therapies prior to TKI, and the starting dose of IM. There were more pts with intermediate and high Sokal risk scores in Group A (p<0.001), and pts in Group A had significantly more comorbidities (p<0.001) with higher CCI scores (p<0.001). Median time of IM exposure (p<0.001) and follow-up durations (p<0.001) were significantly longer in Group B than those of Group A. There were significantly more hematologic AEs among pts in Group A than those of Group B (26% vs. 10%, p=0.01). Nonhematologic AEs were significantly more common in Group A (20% vs. 7%, p=0.022), and the rates of pts with IM dose reduction due to AEs were significantly higher in Group A than that of Group B (28% vs. 11%, p=0.006). Cumulative complete cytogenetic and major molecular response rates and the percentage of patients who switched to second-generation TKIs were similar in both groups (Table 1). Event-free (Fig.1A) and overall (OS) (Fig.1B) survival rates were significantly higher in Group B than those of Group A (p=0.005 and p<0.001, significantly). There were 7 and five pts who died during the follow-up in Groups A and B, respectively. Among these deaths, 5 pts in Group A and two pts in Group B died due to non-CML related causes, and the OS rates were comparable when non-CML related deaths were excluded (Fig.1C) (p=0.096).
Conclusion
Although TKI therapy is relatively safe and effective in elderly pts with CML-CP, TKI-related AEs are more common in this population and comorbidities may play a role in the generation of TKI toxicities and outcomes. Prompt and timely management of these AEs and TKI dose modifications may lead to better outcomes. In our patient cohort, inferior OS rates were observed among elderly pts, but OS rates were similar in both groups when non-CML related deaths were excluded.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Elderly, imatinib, toxicity
Abstract: PB1929
Type: Publication Only
Background
Tyrosine kinase inhibitors (TKIs) are the mainstay of chronic myeloid leukemia (CML) treatment. Median age of CML at diagnosis is 50 years, but a significant proportion of the patients (pts) are diagnosed after age 60. Pts with CML in chronic phase (CML-CP) nowadays live near-normal lifespans, thus, the number of elderly CML pts with comorbidities started to increase.This brings out the issues regarding TKI toxicities, medication adherence and responses to TKI therapy, and we have previously presented the real-life data regarding toxicity and efficacy of imatinib (IM) in our elderly CML cohort [Blood. 2016;128:1905].
Aims
The aim of this study is to update the previously presented data showing the efficacy and safety of IM in the elderly population (pts≥60 years; Group A) with CML and to compare these data with younger patients (pts<60 years; Group B).
Methods
Patient demographics, dose and duration of IM therapy, disease risk scores, cytogenetic and molecular responses, comorbidities, adverse events (AEs), follow-up durations and outcomes were evaluated from pts' files, retrospectively. The Charlson Comorbidity Index (CCI) of each patient was calculated as stated before [Haematologica. 2011;96(10):1457-61].
Results
Our cohort consisted of 181 pts with a median age of 46 years (range, 18-83 years). Group A consisted of 39 pts, and there were 142 pts in Group B (Table 1). The two groups were balanced regarding gender, disease stage, therapies prior to TKI, and the starting dose of IM. There were more pts with intermediate and high Sokal risk scores in Group A (p<0.001), and pts in Group A had significantly more comorbidities (p<0.001) with higher CCI scores (p<0.001). Median time of IM exposure (p<0.001) and follow-up durations (p<0.001) were significantly longer in Group B than those of Group A. There were significantly more hematologic AEs among pts in Group A than those of Group B (26% vs. 10%, p=0.01). Nonhematologic AEs were significantly more common in Group A (20% vs. 7%, p=0.022), and the rates of pts with IM dose reduction due to AEs were significantly higher in Group A than that of Group B (28% vs. 11%, p=0.006). Cumulative complete cytogenetic and major molecular response rates and the percentage of patients who switched to second-generation TKIs were similar in both groups (Table 1). Event-free (Fig.1A) and overall (OS) (Fig.1B) survival rates were significantly higher in Group B than those of Group A (p=0.005 and p<0.001, significantly). There were 7 and five pts who died during the follow-up in Groups A and B, respectively. Among these deaths, 5 pts in Group A and two pts in Group B died due to non-CML related causes, and the OS rates were comparable when non-CML related deaths were excluded (Fig.1C) (p=0.096).

Conclusion
Although TKI therapy is relatively safe and effective in elderly pts with CML-CP, TKI-related AEs are more common in this population and comorbidities may play a role in the generation of TKI toxicities and outcomes. Prompt and timely management of these AEs and TKI dose modifications may lead to better outcomes. In our patient cohort, inferior OS rates were observed among elderly pts, but OS rates were similar in both groups when non-CML related deaths were excluded.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Elderly, imatinib, toxicity


