
Contributions
Abstract: PB1930
Type: Publication Only
Background
Imatinib and the newer BCR-ABL tyrosine kinase inhibitors (TKIs) are the standard therapy for chronic myeloid leukemia (CML). With these drugs, CML patients are achieving similar survivals than the general population, thus classical aspects of chronic diseases, such as treatment adherence and drug-to-drug interactions (DDI) are becoming more important in patients management. DDIs between TKIs and some concurrent medications could lead to toxicity or inadequate response. Although this is a well known effect, and different guidelines include DDIs as a potencial cause of toxicity or resistance, the information about its frequency and its clinical impact is limited.
Aims
To determine the potential DDIs in CML patients treated with TKIs and its clinical impact.
Methods
This was a retrospective, collaborative study performed in 15 centers within the framework of the Spanish CML Group (GELMC). Each participating center included data from all new CML patients that were diagnosed between 1st January 2014 and 31st December 2015, treated with TKI as first-line therapy for CML in chronic or accelerated phase. Concurrent medications, adverse events (AEs), potential DDI and its potencial effects were analyzed.
Results
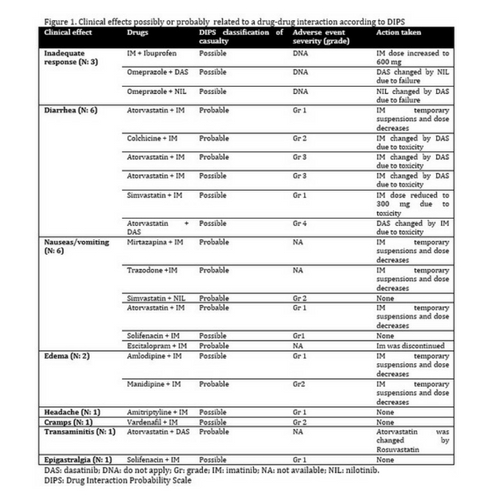
A total of 134 TKI treatments, in 105 patients were included. The mean number of concomitant medications was 4,8 (0-19). The mean number of AEs during the first year of treatment was 2 (SD: 1.9, range 0-11). The AEs severity, according to common terminology criteria for adverse events (CTCAE) 4.03 version, was: grade 1, 40,7%; grade 2, 35,2%; grade 3, 16,1%; and grade 4, 4,8%. Sixtythree patients (60%) had at least one DDI. The mean number of DDIs by TKI treatment was 1,2 (0-8). It was significantly associated with the number of concomitant medications and age. A total of 159 DDIs were detected, involving 55 different drugs, being the most common types, proton pump inhibitors, statins and antidepressants. Clinical or analitical effects of DDIs were suspected by the investigators in only five patients (4,7%). This number increased to 20% in a central review. When such an association was suspected, we applied the DIPS scale (Drug Interaction Probability Scale) to try to estimate causality. We detected 21 clinical effects in 21 patients (20%), that according to the DIPS scale were possibly or probably related to a DDI: 18 (86%) of these effects were related to toxicity, and 3 (14%) to inadequate response (Figure 1). Most of these AEs attributed to DDIs were mild. The most common were diarrhea, vomiting, edema, cramps and transaminitis, and 78,5% were grade 1-2. We did not find significant differences in the frequency of AEs, or in the molecular response, in patients with or without DDIs.
Conclusion
Potential DDIs are present in most of patients treated with TKIs. A clinical effect was suspected by the treating phisycians in only 4.7% of the patients, but increased to 20% in the central review. Nevertheless most of this possible or probable clinical effects were mild, and could possibly had appeared with the individual drugs, thus it is difficult to be sure to what extent the DDIs have caused or worsened the AEs. We did not see a clear effect of DDI in response as a group, although 3 patients with inadequate response were taking drugs that could decrease TKI effectivity. Thus, due to DDIs high frequency, and the possibility of clinical relevant effects, we consider that physicians treating CML patients should consider this aspect in their patients management.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Adverse reaction, Chronic myeloid leukemia, Tyrosine kinase inhibitor
Abstract: PB1930
Type: Publication Only
Background
Imatinib and the newer BCR-ABL tyrosine kinase inhibitors (TKIs) are the standard therapy for chronic myeloid leukemia (CML). With these drugs, CML patients are achieving similar survivals than the general population, thus classical aspects of chronic diseases, such as treatment adherence and drug-to-drug interactions (DDI) are becoming more important in patients management. DDIs between TKIs and some concurrent medications could lead to toxicity or inadequate response. Although this is a well known effect, and different guidelines include DDIs as a potencial cause of toxicity or resistance, the information about its frequency and its clinical impact is limited.
Aims
To determine the potential DDIs in CML patients treated with TKIs and its clinical impact.
Methods
This was a retrospective, collaborative study performed in 15 centers within the framework of the Spanish CML Group (GELMC). Each participating center included data from all new CML patients that were diagnosed between 1st January 2014 and 31st December 2015, treated with TKI as first-line therapy for CML in chronic or accelerated phase. Concurrent medications, adverse events (AEs), potential DDI and its potencial effects were analyzed.
Results
A total of 134 TKI treatments, in 105 patients were included. The mean number of concomitant medications was 4,8 (0-19). The mean number of AEs during the first year of treatment was 2 (SD: 1.9, range 0-11). The AEs severity, according to common terminology criteria for adverse events (CTCAE) 4.03 version, was: grade 1, 40,7%; grade 2, 35,2%; grade 3, 16,1%; and grade 4, 4,8%. Sixtythree patients (60%) had at least one DDI. The mean number of DDIs by TKI treatment was 1,2 (0-8). It was significantly associated with the number of concomitant medications and age. A total of 159 DDIs were detected, involving 55 different drugs, being the most common types, proton pump inhibitors, statins and antidepressants. Clinical or analitical effects of DDIs were suspected by the investigators in only five patients (4,7%). This number increased to 20% in a central review. When such an association was suspected, we applied the DIPS scale (Drug Interaction Probability Scale) to try to estimate causality. We detected 21 clinical effects in 21 patients (20%), that according to the DIPS scale were possibly or probably related to a DDI: 18 (86%) of these effects were related to toxicity, and 3 (14%) to inadequate response (Figure 1). Most of these AEs attributed to DDIs were mild. The most common were diarrhea, vomiting, edema, cramps and transaminitis, and 78,5% were grade 1-2. We did not find significant differences in the frequency of AEs, or in the molecular response, in patients with or without DDIs.

Conclusion
Potential DDIs are present in most of patients treated with TKIs. A clinical effect was suspected by the treating phisycians in only 4.7% of the patients, but increased to 20% in the central review. Nevertheless most of this possible or probable clinical effects were mild, and could possibly had appeared with the individual drugs, thus it is difficult to be sure to what extent the DDIs have caused or worsened the AEs. We did not see a clear effect of DDI in response as a group, although 3 patients with inadequate response were taking drugs that could decrease TKI effectivity. Thus, due to DDIs high frequency, and the possibility of clinical relevant effects, we consider that physicians treating CML patients should consider this aspect in their patients management.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Adverse reaction, Chronic myeloid leukemia, Tyrosine kinase inhibitor


