
Contributions
Abstract: PB1888
Type: Publication Only
Background
Ibrutinib is an irreversible molecular inhibitor of Bruton's tyrosine kinase (ITK), which has altered the treatment paradigm for hematological diseases such as Chronic Lymphocytic Leukemia (CLL), Mantle Cell Lymphoma (MCL), and Waldeström's Macroglobulinemia (WM). Due to its recent incorporation, long-term safety profile has not yet been defined, although the most frequently reported adverse effects are the following: diarrhea, neutropenia, mild bleeding, exanthema and musculoskeletal pain.
Aims
Our aim is to analyze Ibrutinib’s adverse effects in our group of patients, paying special attention to cardiological, infectious and hemorrhagic ones.
Methods
It is a descriptive, observational and retrospective study on the use of Ibrutinib in our hospital between February 2016 and February 2018. We analyzed patient’s profile (age, sex, previous cardiological disease and anticoagulant and antiplatelet therapy, hematological diagnosis, and previous treatment lines), duration of Ibrutinib-based therapy and level of response achieved, adverse effects, treatment interruptions and dose reduction (if needed).
Results
Thirty-one patients were treated with Ibrutinib, of which 74% were male and 26% female, with an average age of 70.5 years (between 48-84). Of the total, four had previous heart disease: Atrial Fibrillation (2), ischemic heart disease (1), and aortic valve insufficiency (1); three were being treated with acetylsalicylic acid (ASA), one with ASA and Clopidogrel, and two with oral anticoagulants (Acenocoumarol/Apixaban). ASA was suspended on the first three cases and Clopidogrel on the fourth one.
Treatment indications were the following: CLL (17), MCL (7), WM (4), B-Cell Prolymphocytic Leukemia (2) and Diffuse Large B Cell Lymphoma (1). Of the 31 patients, 8 received Ibrutinib as first-line treatment, 17 as second-line, 4 as third-line and 2 as fourth-line. The average duration of treatment was 7 months (between 0.25-21.5). Inicial dose had to be reduced in one patient due to mild hepatic failure (Child-Pugh A).
In terms of response level, 10 achieved complete response, 4 partial response (of which one later progressed), 7 progressed during treatment (4 MCL -as second line-, 3 CLL -as second line-, and 1 B Cell Prolymphocytic Leukemia -as first line-). In the 9 remaining patients, the level of response has not yet been evaluated since they have recently started therapy with Ibrutinib.
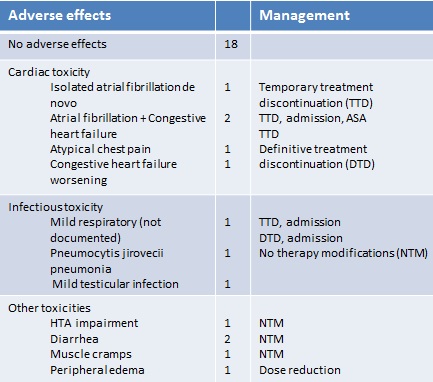
Adverse effects observed during the treatment period are detailed in the chart (see attached file 1).
Conclusion
Based on our experience, the use of Ibrutinib has an acceptable toxicity profile, with adverse cardiac effects being the most frequent and serious. For this reason, we consider fundamental previous cardiological assessment of the patients and their close monitoring during the treatment. However, it seems important to note that the majority of adverse effects have occurred in patients in second and third lines of treatment.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Chronic Lymphocytic Leukemia, ibrutinib, Mantle cell lymphoma, Waldenstrom's macroglobulinemia
Abstract: PB1888
Type: Publication Only
Background
Ibrutinib is an irreversible molecular inhibitor of Bruton's tyrosine kinase (ITK), which has altered the treatment paradigm for hematological diseases such as Chronic Lymphocytic Leukemia (CLL), Mantle Cell Lymphoma (MCL), and Waldeström's Macroglobulinemia (WM). Due to its recent incorporation, long-term safety profile has not yet been defined, although the most frequently reported adverse effects are the following: diarrhea, neutropenia, mild bleeding, exanthema and musculoskeletal pain.
Aims
Our aim is to analyze Ibrutinib’s adverse effects in our group of patients, paying special attention to cardiological, infectious and hemorrhagic ones.
Methods
It is a descriptive, observational and retrospective study on the use of Ibrutinib in our hospital between February 2016 and February 2018. We analyzed patient’s profile (age, sex, previous cardiological disease and anticoagulant and antiplatelet therapy, hematological diagnosis, and previous treatment lines), duration of Ibrutinib-based therapy and level of response achieved, adverse effects, treatment interruptions and dose reduction (if needed).
Results
Thirty-one patients were treated with Ibrutinib, of which 74% were male and 26% female, with an average age of 70.5 years (between 48-84). Of the total, four had previous heart disease: Atrial Fibrillation (2), ischemic heart disease (1), and aortic valve insufficiency (1); three were being treated with acetylsalicylic acid (ASA), one with ASA and Clopidogrel, and two with oral anticoagulants (Acenocoumarol/Apixaban). ASA was suspended on the first three cases and Clopidogrel on the fourth one.
Treatment indications were the following: CLL (17), MCL (7), WM (4), B-Cell Prolymphocytic Leukemia (2) and Diffuse Large B Cell Lymphoma (1). Of the 31 patients, 8 received Ibrutinib as first-line treatment, 17 as second-line, 4 as third-line and 2 as fourth-line. The average duration of treatment was 7 months (between 0.25-21.5). Inicial dose had to be reduced in one patient due to mild hepatic failure (Child-Pugh A).
In terms of response level, 10 achieved complete response, 4 partial response (of which one later progressed), 7 progressed during treatment (4 MCL -as second line-, 3 CLL -as second line-, and 1 B Cell Prolymphocytic Leukemia -as first line-). In the 9 remaining patients, the level of response has not yet been evaluated since they have recently started therapy with Ibrutinib.
Adverse effects observed during the treatment period are detailed in the chart (see attached file 1).

Conclusion
Based on our experience, the use of Ibrutinib has an acceptable toxicity profile, with adverse cardiac effects being the most frequent and serious. For this reason, we consider fundamental previous cardiological assessment of the patients and their close monitoring during the treatment. However, it seems important to note that the majority of adverse effects have occurred in patients in second and third lines of treatment.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Chronic Lymphocytic Leukemia, ibrutinib, Mantle cell lymphoma, Waldenstrom's macroglobulinemia


