
Contributions
Abstract: PB1863
Type: Publication Only
Background
It is generally accepted that CLL patients with late disease progression after chemoimmunotherapy (CIT) remain chemosensitive and can be successfully retreated with the same regimen if they are still “fit” and no del17p/TP53mut is acquired. Accordingly, newer treatment options, such as Btk and PI3K inhibitors, are reserved to patients with so called “early relapse”. As switch to newer drugs is often a difficult medical decision with consequences both to patients (different toxicity profile) and economy (higher treatment costs), a stringent evidence-based definition of “early relapse” is desirable.
Aims
We tried to analyze consistency and evidence base in the proposed thresholds of “early relapse” in different publicly available CLL guidelines.
Methods
PubMed and Google were searched for terms “CLL guideline” and “CLL recommendation”. Relevant documents were collected from search results and analyzed for clinical guidance and references.
Results
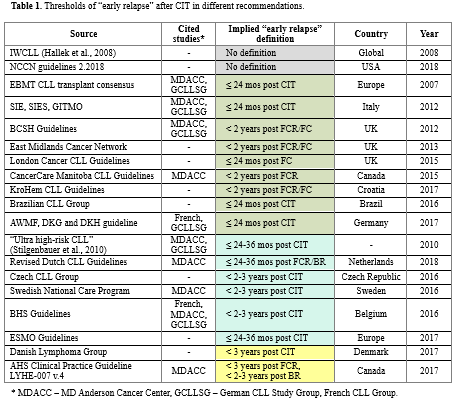
Results are summarized in Table 1. All documents specified different timings of “early relapse” (namely, within 24, 24-36 or 36 mos after CIT). In two documents relevant clinical guidance was marked with II-III evidence level, however no prospective trials were cited with time-of-relapse based interventions or prespecified outcome analysis. Some guidelines didn’t provide rational for a particular choice of threshold at all. Those documents, that gave references, cited MDACC (Keating et al., 1998; Keating et al. 2005; Tam et al., 2008; Keating et al., 2009; Tam et al., 2014), GCLLSG (Stilgenbauer et al., 2010; Cramer et al., 2015) or French Group (Fornecker et al., 2015). However, these sources appear to have serious limitations. For example, in publications from MDACC overall survival difference was calculated for a threshold of 36 mos from the start of FCR (that is, approx. 30 mos post CIT). Same issue applies to CLL8 data provided in Stilgenbauer et al., 2010 (PFS of 24 mos, approx. 18 mos post CIT). Hence, thresholds of 24 or 36 mos after CIT appear to be not properly validated. Also, large meta-analysis of 1558 pts by Cramer et al. 2015 didn’t find statistically significant difference in OS at 1, 2 or 3 year thresholds and French Group results are based on relatively small (n =132) retrospective cohort. Additionally, although all citied studies analyzed prognostic role of early relapses solely after 1st line CIT, none of the guidelines mentioned the fact that suggested thresholds can’t be extrapolated to relapses after further lines of therapy.
Conclusion
There is a considerable variability and paucity of evidence base in the thresholds for “early relapse” proposed by current CLL guidelines. This can potentially impede clinical practice, leading to untimely or/and unjustified therapeutic decisions when switch to newer inhibitors and allogeneic stem cell transplantation is considered. Also, a question remains on which duration of remission can sufficiently predict sensitivity to CIT in the context of subsequent relapses. Analysis of large prospective datasets is needed.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Chemosensitivity, Chronic Lymphocytic Leukemia
Abstract: PB1863
Type: Publication Only
Background
It is generally accepted that CLL patients with late disease progression after chemoimmunotherapy (CIT) remain chemosensitive and can be successfully retreated with the same regimen if they are still “fit” and no del17p/TP53mut is acquired. Accordingly, newer treatment options, such as Btk and PI3K inhibitors, are reserved to patients with so called “early relapse”. As switch to newer drugs is often a difficult medical decision with consequences both to patients (different toxicity profile) and economy (higher treatment costs), a stringent evidence-based definition of “early relapse” is desirable.
Aims
We tried to analyze consistency and evidence base in the proposed thresholds of “early relapse” in different publicly available CLL guidelines.
Methods
PubMed and Google were searched for terms “CLL guideline” and “CLL recommendation”. Relevant documents were collected from search results and analyzed for clinical guidance and references.
Results
Results are summarized in Table 1. All documents specified different timings of “early relapse” (namely, within 24, 24-36 or 36 mos after CIT). In two documents relevant clinical guidance was marked with II-III evidence level, however no prospective trials were cited with time-of-relapse based interventions or prespecified outcome analysis. Some guidelines didn’t provide rational for a particular choice of threshold at all. Those documents, that gave references, cited MDACC (Keating et al., 1998; Keating et al. 2005; Tam et al., 2008; Keating et al., 2009; Tam et al., 2014), GCLLSG (Stilgenbauer et al., 2010; Cramer et al., 2015) or French Group (Fornecker et al., 2015). However, these sources appear to have serious limitations. For example, in publications from MDACC overall survival difference was calculated for a threshold of 36 mos from the start of FCR (that is, approx. 30 mos post CIT). Same issue applies to CLL8 data provided in Stilgenbauer et al., 2010 (PFS of 24 mos, approx. 18 mos post CIT). Hence, thresholds of 24 or 36 mos after CIT appear to be not properly validated. Also, large meta-analysis of 1558 pts by Cramer et al. 2015 didn’t find statistically significant difference in OS at 1, 2 or 3 year thresholds and French Group results are based on relatively small (n =132) retrospective cohort. Additionally, although all citied studies analyzed prognostic role of early relapses solely after 1st line CIT, none of the guidelines mentioned the fact that suggested thresholds can’t be extrapolated to relapses after further lines of therapy.

Conclusion
There is a considerable variability and paucity of evidence base in the thresholds for “early relapse” proposed by current CLL guidelines. This can potentially impede clinical practice, leading to untimely or/and unjustified therapeutic decisions when switch to newer inhibitors and allogeneic stem cell transplantation is considered. Also, a question remains on which duration of remission can sufficiently predict sensitivity to CIT in the context of subsequent relapses. Analysis of large prospective datasets is needed.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Chemosensitivity, Chronic Lymphocytic Leukemia


