
Contributions
Abstract: PB1886
Type: Publication Only
Background
Despite the success of CLL treatment with fludarabine and bendamustine-containing regimens, about 25% of patients had relapsed within the first 24 monthsafter the end of the first-line therapy.In this group of patients PFS after standard “salvage therapy” is 6-18 months,overall survival is extremely low – 13-47 months.???The lack of effective approaches to second-line therapy for patients with earlyrelapses of CLL has led to the application of new agent in clinical practice -ibrutinib. Despite the success of CLL treatment with fludarabine and bendamustine-containing regimens, about 25% of patients had relapsed within the first 24 monthsafter the end of the first-line therapy. In this group of patients PFS after standard “salvage therapy” is 6-18 months, overall survival is extremely low – 13-47 months. The lack of effective approaches to second-line therapy for patients with earlyrelapses of CLL has led to the application of new agent in clinical practice - ibrutinib.
Aims
To estimate ibrutinib efficacy in the treatment of early CLL relapses and in patients with ≥ 2 lines of preceding therapy. Analysis of treatment results in patients with del(17p) and monitoring of minimal residual disease (MRD) and ibrutinib safety profile.
Methods
The analysis included the results of ibrutinib treatment in 31 patients with CLL. Twenty eight patients were treated by bendamustine and fludarabine containing regimens. The median prior treatment lines were 2 (range 1–10). The indications for the treatment initiation were the fi rst early relapse in 51 % of cases (n = 16) and a relapse after 2 and more lines of therapy in 49 % of cases (n = 15). Ibrutinib was administered in mono- (n = 15) and combined therapy (n = 14) as well as in the R-BAC scheme (n = 2). Using FISH analysis del(17p) was found in 9 patients (34 %).
Results
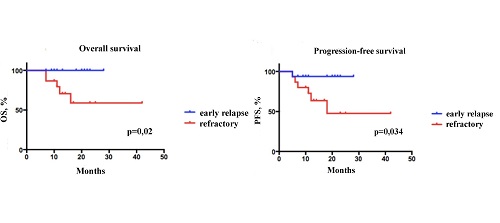
Within the median follow up of 18 months (range 7–42+) the overall survival (OS) rate was reported to be 87 %, and the progression-free survival (PFS) rate was 7 %. The maximum MRD after a year of ibrutinib treatment was observed in case of combination with immunochemotherapy (e.g., R-BAC). Within the period of 18 months OS rate was 100 %, in the patient group with early relapses and 66 % in the group with a relapse after 2 and more therapy lines (p = 0.02). Within the same examination period PFS was signifi cantly higher (94 %) in the patient group with early relapses compared to the previously treated patients (60 %) (p = 0.034). The most common adverse events were grade 1–2 purpura (30 %), grade 1–2 diarrhea (10 %), atrial fi brillation paroxysms (10 %) and arterial hypertension (10 %). Severe infectious complications registered in 6 % (n = 3) patients were successfully solved in the course of combined antibacterial and antimycotic treatment
Conclusion
Ibrutinib was shown to be effective drug for treatment of relapsed CLL. The OS and PFS values were more favourable in patients with early relapses compared to the patients with relapses after ≥ 2 lines of therapy prior to ibrutinib treatment. The maximum elimination of the tumor clone was observed after combined ibrutinib/immunochemotherapy treatment. The tolerance of ibrutinib was reported to be satisfactory with acceptable toxicity profile. No mortality due to infection complications was observed.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Abstract: PB1886
Type: Publication Only
Background
Despite the success of CLL treatment with fludarabine and bendamustine-containing regimens, about 25% of patients had relapsed within the first 24 monthsafter the end of the first-line therapy.In this group of patients PFS after standard “salvage therapy” is 6-18 months,overall survival is extremely low – 13-47 months.???The lack of effective approaches to second-line therapy for patients with earlyrelapses of CLL has led to the application of new agent in clinical practice -ibrutinib. Despite the success of CLL treatment with fludarabine and bendamustine-containing regimens, about 25% of patients had relapsed within the first 24 monthsafter the end of the first-line therapy. In this group of patients PFS after standard “salvage therapy” is 6-18 months, overall survival is extremely low – 13-47 months. The lack of effective approaches to second-line therapy for patients with earlyrelapses of CLL has led to the application of new agent in clinical practice - ibrutinib.
Aims
To estimate ibrutinib efficacy in the treatment of early CLL relapses and in patients with ≥ 2 lines of preceding therapy. Analysis of treatment results in patients with del(17p) and monitoring of minimal residual disease (MRD) and ibrutinib safety profile.
Methods
The analysis included the results of ibrutinib treatment in 31 patients with CLL. Twenty eight patients were treated by bendamustine and fludarabine containing regimens. The median prior treatment lines were 2 (range 1–10). The indications for the treatment initiation were the fi rst early relapse in 51 % of cases (n = 16) and a relapse after 2 and more lines of therapy in 49 % of cases (n = 15). Ibrutinib was administered in mono- (n = 15) and combined therapy (n = 14) as well as in the R-BAC scheme (n = 2). Using FISH analysis del(17p) was found in 9 patients (34 %).
Results
Within the median follow up of 18 months (range 7–42+) the overall survival (OS) rate was reported to be 87 %, and the progression-free survival (PFS) rate was 7 %. The maximum MRD after a year of ibrutinib treatment was observed in case of combination with immunochemotherapy (e.g., R-BAC). Within the period of 18 months OS rate was 100 %, in the patient group with early relapses and 66 % in the group with a relapse after 2 and more therapy lines (p = 0.02). Within the same examination period PFS was signifi cantly higher (94 %) in the patient group with early relapses compared to the previously treated patients (60 %) (p = 0.034). The most common adverse events were grade 1–2 purpura (30 %), grade 1–2 diarrhea (10 %), atrial fi brillation paroxysms (10 %) and arterial hypertension (10 %). Severe infectious complications registered in 6 % (n = 3) patients were successfully solved in the course of combined antibacterial and antimycotic treatment

Conclusion
Ibrutinib was shown to be effective drug for treatment of relapsed CLL. The OS and PFS values were more favourable in patients with early relapses compared to the patients with relapses after ≥ 2 lines of therapy prior to ibrutinib treatment. The maximum elimination of the tumor clone was observed after combined ibrutinib/immunochemotherapy treatment. The tolerance of ibrutinib was reported to be satisfactory with acceptable toxicity profile. No mortality due to infection complications was observed.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical


