
Contributions
Abstract: PB1848
Type: Publication Only
Background
CLL is an incurable lymphoproliferative disease where treatment landscape has rapidly evolved with the approval of several novel oral targeted therapies across various settings. Most settings are unique based on baseline risk profiles of the patients (pts). In absence of head-to-head trials, it is challenging to compare treatments for researchers and physicians as different treatments are approved in different patient segments. There are important prognostic and predictive parameters that guide the therapy choices and outcomes. Using appropriate methods, CLL can be classified into three risk groups differing in efficacy and safety outcomes that can guide patient selection for specific therapies.
Aims
The primary aim of the study is to evaluate risk adjusted relative scores of efficacy and safety for approved drugs in CLL.
Methods
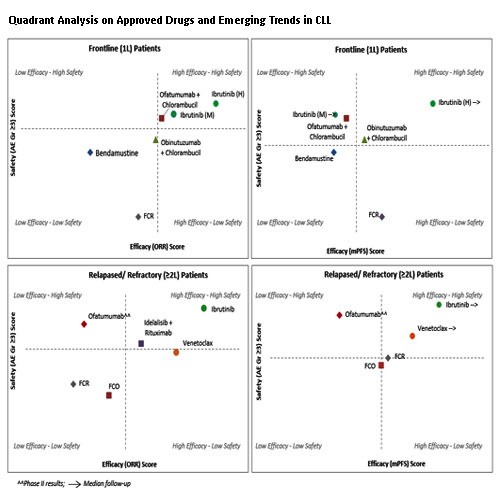
The study used data from SMARTOncology Database (SmartAnalyst Inc., USA) developed for internal research purposes using public sources such as ClinicalTrials.gov, publications, company websites and FDA labels. The study derived patient risk scores based on prognostic variables such as median prior lines of therapy, ECOG, staging, co-morbidities to categorize pts into low, medium and high-risk groups. This study also considered pts with 17p deletion (del)/TP53 mutations (mut) as high-risk pts. For the trials where 17p del/TP53 mut information was missing, the risk categorization was done based on above prognostic factors. The risk-adjusted relative scores of efficacy and safety endpoints (ORR, mPFS and grade ≥3AEs) were derived based on patient risk scores to design the quadrants. The individual quadrants were designed for ORR and safety endpoints and for mPFS and safety endpoints. The X-axis represented efficacy from ‘Low-High’ and the Y-axis represented safety from ‘Low-High’. The adjusted relative score for each drug was plotted and collectively rated based on the position of the drug in the respective quadrant. The drugs represented in the quadrant of ‘High efficacy’ and ‘High safety’ can also be called as “quadrant of effectiveness”. The analysis was carried out separately for the drugs approved in frontline and relapsed/refractory (RR) settings.
Results
The study revealed that Ibrutinib and Ofatumumab+chlorambucil were observed in the quadrant of effectiveness based on ORR and safety endpoints, while Ibrutinib and Obinutuzumab +chlorambucil were observed in the quadrant of effectiveness based on mPFS and safety endpoints in frontline. The study revealed that Ibrutinib has the most promising efficacy and safety as compared to other novel drugs. In RR setting, Ibrutinib and Venetoclax were observed in the quadrant of effectiveness based on both ORR and safety endpoints as well as mPFS and safety endpoints. While Idelalisib+Rituximab represented relatively lower effectiveness compared to the other drugs. The study also revealed that Ibrutinib and Venetoclax were the most promising drugs in RR setting compared to others.
Conclusion
Despite multiple approvals of novel therapies in CLL, unmet need still persists. These approved therapies may be tested in wide range of patient settings. The current analysis suggests that if the therapies were observed in the quadrant of effectiveness the probability of approval may increase and so does the broader applicability across multiple settings. Further analysis will help in understanding the correlation of differences in patient characteristics and cytogenetic testing practices on treatment decisions and outcomes.
Session topic: 5. Chronic lymphocytic leukemia and related disorders – Biology & Translational Research
Keyword(s): Chronic Lymphocytic Leukemia, Risk factor, Targeted therapy
Abstract: PB1848
Type: Publication Only
Background
CLL is an incurable lymphoproliferative disease where treatment landscape has rapidly evolved with the approval of several novel oral targeted therapies across various settings. Most settings are unique based on baseline risk profiles of the patients (pts). In absence of head-to-head trials, it is challenging to compare treatments for researchers and physicians as different treatments are approved in different patient segments. There are important prognostic and predictive parameters that guide the therapy choices and outcomes. Using appropriate methods, CLL can be classified into three risk groups differing in efficacy and safety outcomes that can guide patient selection for specific therapies.
Aims
The primary aim of the study is to evaluate risk adjusted relative scores of efficacy and safety for approved drugs in CLL.
Methods
The study used data from SMARTOncology Database (SmartAnalyst Inc., USA) developed for internal research purposes using public sources such as ClinicalTrials.gov, publications, company websites and FDA labels. The study derived patient risk scores based on prognostic variables such as median prior lines of therapy, ECOG, staging, co-morbidities to categorize pts into low, medium and high-risk groups. This study also considered pts with 17p deletion (del)/TP53 mutations (mut) as high-risk pts. For the trials where 17p del/TP53 mut information was missing, the risk categorization was done based on above prognostic factors. The risk-adjusted relative scores of efficacy and safety endpoints (ORR, mPFS and grade ≥3AEs) were derived based on patient risk scores to design the quadrants. The individual quadrants were designed for ORR and safety endpoints and for mPFS and safety endpoints. The X-axis represented efficacy from ‘Low-High’ and the Y-axis represented safety from ‘Low-High’. The adjusted relative score for each drug was plotted and collectively rated based on the position of the drug in the respective quadrant. The drugs represented in the quadrant of ‘High efficacy’ and ‘High safety’ can also be called as “quadrant of effectiveness”. The analysis was carried out separately for the drugs approved in frontline and relapsed/refractory (RR) settings.
Results
The study revealed that Ibrutinib and Ofatumumab+chlorambucil were observed in the quadrant of effectiveness based on ORR and safety endpoints, while Ibrutinib and Obinutuzumab +chlorambucil were observed in the quadrant of effectiveness based on mPFS and safety endpoints in frontline. The study revealed that Ibrutinib has the most promising efficacy and safety as compared to other novel drugs. In RR setting, Ibrutinib and Venetoclax were observed in the quadrant of effectiveness based on both ORR and safety endpoints as well as mPFS and safety endpoints. While Idelalisib+Rituximab represented relatively lower effectiveness compared to the other drugs. The study also revealed that Ibrutinib and Venetoclax were the most promising drugs in RR setting compared to others.

Conclusion
Despite multiple approvals of novel therapies in CLL, unmet need still persists. These approved therapies may be tested in wide range of patient settings. The current analysis suggests that if the therapies were observed in the quadrant of effectiveness the probability of approval may increase and so does the broader applicability across multiple settings. Further analysis will help in understanding the correlation of differences in patient characteristics and cytogenetic testing practices on treatment decisions and outcomes.
Session topic: 5. Chronic lymphocytic leukemia and related disorders – Biology & Translational Research
Keyword(s): Chronic Lymphocytic Leukemia, Risk factor, Targeted therapy


