
Contributions
Abstract: PB1854
Type: Publication Only
Background
Personalized cancer therapy is a rapidly developing field that includes predictive medicine, preventive medicine and various personalized or individualized therapies, e.g. labeled “precision medicine”. One particular challenge with cancer is tumor heterogeneity evolving after the original clonal event. We focus on Chronic Lymphocytic Leukemia (CLL), Multiple Myeloma and Follicular lymphoma (FL), diseases currently considered incurable. Although current treatment regimens prolong life for patients, CLL eventually relapse. Current potential in using therapeutics against CLL include design of optimal treatment for individual patients based on characterization of the tumor and its intratumor heterogeneity as observed by whole genome sequencing. Efficient therapies require a personalized approach that combines targeting cancer cells and the tumor microenvironment by restoring the patient’s own anti-tumor immunity. Another major limitation is that there exists no efficient approach to identify the most efficient drugs for each patient and also for different cancer stage. Using our drug sensitivity screening platform, we aim to address the limitation in identifying the efficient drugs for individual patients.
Aims
To introduce individualized treatment for patients, we aim to establish cell-based assays and a drug sensitivity platform. We aim to establish a pipeline for drug sensitivity screening in CLL. To complement the results from the drug sensitivity screening, we aim to perform phosphoflow analysis. We aim to complement our approach using xenografted mice. We propose to use the drug sensitivity screening platform for CLL individualized treatment with an effective combination of targeted therapies.
Methods
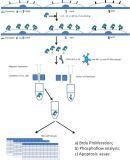
To define drugs that inhibit malignant B cell growth, we use the cell-based assays CellTiter-Glo® luminescent cell viability assay and CellTox™ green cytotoxicity assay. We have established culture settings that mimic the tumor microenvironment for CLL. We perform drug sensitivity screening with 517 drugs at 5 concentrations to select drug candidates and pathway inhibitors. Selected drug candidates will be further analyzed by bioassays and flow cytometry to assess effects on intracellular signaling (phosphoflow-based approach). We propose to use the drug sensitivity screening platform to identify and validate drug candidates for xenografting and precision medicine clinical trials.
Results
We established an experimental setting that mimics the tumor microenvironment for CLL (Thimiri Govinda Raj et al EHA meeting abstract 2017 Haematologica 102, 711-711). We have performed drug screening on 20 patient samples with 517 drugs at 5 concentrations and are currently aggregating data and expanding the data set. We have also performed drug screening on healthy B cells as a control.
Conclusion
Using our established CLL drug sensitivity screening at NCMM, we have performed drug screening on a number of patient samples. We aim to use a statistical approach to identify synergistic drug effects and validate the drug combinations experimentally. As a future perspective, we would combine machine learning strategies with the experimental drug screening strategies for the precision medicine clinical trials in other cancer settings.
Session topic: 5. Chronic lymphocytic leukemia and related disorders – Biology & Translational Research
Keyword(s): B cell chronic lymphocytic leukemia, Chronic Lymphocytic Leukemia, Drug resistance, Drug sensitivity
Abstract: PB1854
Type: Publication Only
Background
Personalized cancer therapy is a rapidly developing field that includes predictive medicine, preventive medicine and various personalized or individualized therapies, e.g. labeled “precision medicine”. One particular challenge with cancer is tumor heterogeneity evolving after the original clonal event. We focus on Chronic Lymphocytic Leukemia (CLL), Multiple Myeloma and Follicular lymphoma (FL), diseases currently considered incurable. Although current treatment regimens prolong life for patients, CLL eventually relapse. Current potential in using therapeutics against CLL include design of optimal treatment for individual patients based on characterization of the tumor and its intratumor heterogeneity as observed by whole genome sequencing. Efficient therapies require a personalized approach that combines targeting cancer cells and the tumor microenvironment by restoring the patient’s own anti-tumor immunity. Another major limitation is that there exists no efficient approach to identify the most efficient drugs for each patient and also for different cancer stage. Using our drug sensitivity screening platform, we aim to address the limitation in identifying the efficient drugs for individual patients.
Aims
To introduce individualized treatment for patients, we aim to establish cell-based assays and a drug sensitivity platform. We aim to establish a pipeline for drug sensitivity screening in CLL. To complement the results from the drug sensitivity screening, we aim to perform phosphoflow analysis. We aim to complement our approach using xenografted mice. We propose to use the drug sensitivity screening platform for CLL individualized treatment with an effective combination of targeted therapies.
Methods
To define drugs that inhibit malignant B cell growth, we use the cell-based assays CellTiter-Glo® luminescent cell viability assay and CellTox™ green cytotoxicity assay. We have established culture settings that mimic the tumor microenvironment for CLL. We perform drug sensitivity screening with 517 drugs at 5 concentrations to select drug candidates and pathway inhibitors. Selected drug candidates will be further analyzed by bioassays and flow cytometry to assess effects on intracellular signaling (phosphoflow-based approach). We propose to use the drug sensitivity screening platform to identify and validate drug candidates for xenografting and precision medicine clinical trials.
Results
We established an experimental setting that mimics the tumor microenvironment for CLL (Thimiri Govinda Raj et al EHA meeting abstract 2017 Haematologica 102, 711-711). We have performed drug screening on 20 patient samples with 517 drugs at 5 concentrations and are currently aggregating data and expanding the data set. We have also performed drug screening on healthy B cells as a control.

Conclusion
Using our established CLL drug sensitivity screening at NCMM, we have performed drug screening on a number of patient samples. We aim to use a statistical approach to identify synergistic drug effects and validate the drug combinations experimentally. As a future perspective, we would combine machine learning strategies with the experimental drug screening strategies for the precision medicine clinical trials in other cancer settings.
Session topic: 5. Chronic lymphocytic leukemia and related disorders – Biology & Translational Research
Keyword(s): B cell chronic lymphocytic leukemia, Chronic Lymphocytic Leukemia, Drug resistance, Drug sensitivity


