
Contributions
Abstract: PB1738
Type: Publication Only
Background
There are great unmet needs to explore more efficient and low-cytotoxic treatment for refractory or relapsed acute myeloid leukemia (R/R AML) patients. CD117 high expression was reported to be related with higher relapse of AML. But its inhibitors, such as imatinib or dasatinib, were not reported to successfully treat R/R AML by now. Because sorafenib targets multiple tyrosine kinases including CD117, and it has been reported to successfully treat R/R AML patients with FLT3/ITD mutation as a single agent. Here we retrospectively investigated the outcomes of seven R/R patients with high CD117 expression but without FLT3/ITD and C-kit mutation treated with single sorafenib.
Aims
The aims of this study is to see if sorafenib treatment alone could benefit this special group of patients with high CD117 expression but without FLT3/ITD and C-kit mutation.
Methods
Seven R/R AML patients without FLT3-ITD or C-kit mutations who were treated by single sorafenib, 0.4 gram twice a day, were sequentially enrolled in our center from May 2015 to August 2016. CD117 were positively expressed on more than 60% of bone marrow blast cells. Diagnosis was made according to the category criterion of WHO 2008. The risk status assessments and standards of patients’ responses were referred to the NCCN guideline. Side effect grade was named according to common terminology criteria for adverse events v 4.0 by US National Institutes of Health. The outcomes of patients were followed up till September 2017.
Results
Four of the 7 patients achieved CR or CRi with single sorafenib. The time required to remission ranged from 31 to 100 days variably. In other three NR patients, two could also have a temporary decrease of bone marrow blast cells in the first week, but all had increment of blast cells in the latter two to three weeks and prescription of sorafenib went to cease.
During sorafenib induction, all patients experienced grade 3 febrile neutropenia but had no unendurable infections. One patient developed grade 3 palmar-plantar erythrodysesthesia at the day 54. But it tapered in one week after stopping the drug.
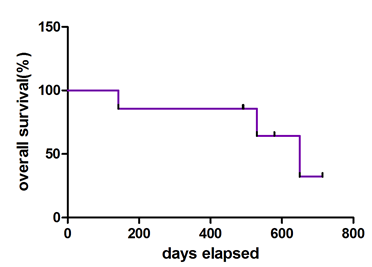
The median follow-up for the whole cohort have been more than 17 months. Three patients passed away for relapse of AML and their event free survival with sorafenib ranged from 2 to 20 months. All four patients who accepted stem cell transplantation were still alive at the end of follow-up no matter they responded to sorafinib or not. The median survival time initiating from sorafenib usage has been 650 days.
Conclusion
The preliminary study on single sorafenib treatment responses of R/R AML with high CD117 expression and no FLT3-ITD mutation gave an encouraging result. Sorafinib, at least as a bridge to transplantation, should be considered in such special R/R AML patients in further study.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, C-kit, Flt3-ITD, Refractory
Abstract: PB1738
Type: Publication Only
Background
There are great unmet needs to explore more efficient and low-cytotoxic treatment for refractory or relapsed acute myeloid leukemia (R/R AML) patients. CD117 high expression was reported to be related with higher relapse of AML. But its inhibitors, such as imatinib or dasatinib, were not reported to successfully treat R/R AML by now. Because sorafenib targets multiple tyrosine kinases including CD117, and it has been reported to successfully treat R/R AML patients with FLT3/ITD mutation as a single agent. Here we retrospectively investigated the outcomes of seven R/R patients with high CD117 expression but without FLT3/ITD and C-kit mutation treated with single sorafenib.
Aims
The aims of this study is to see if sorafenib treatment alone could benefit this special group of patients with high CD117 expression but without FLT3/ITD and C-kit mutation.
Methods
Seven R/R AML patients without FLT3-ITD or C-kit mutations who were treated by single sorafenib, 0.4 gram twice a day, were sequentially enrolled in our center from May 2015 to August 2016. CD117 were positively expressed on more than 60% of bone marrow blast cells. Diagnosis was made according to the category criterion of WHO 2008. The risk status assessments and standards of patients’ responses were referred to the NCCN guideline. Side effect grade was named according to common terminology criteria for adverse events v 4.0 by US National Institutes of Health. The outcomes of patients were followed up till September 2017.
Results
Four of the 7 patients achieved CR or CRi with single sorafenib. The time required to remission ranged from 31 to 100 days variably. In other three NR patients, two could also have a temporary decrease of bone marrow blast cells in the first week, but all had increment of blast cells in the latter two to three weeks and prescription of sorafenib went to cease.
During sorafenib induction, all patients experienced grade 3 febrile neutropenia but had no unendurable infections. One patient developed grade 3 palmar-plantar erythrodysesthesia at the day 54. But it tapered in one week after stopping the drug.
The median follow-up for the whole cohort have been more than 17 months. Three patients passed away for relapse of AML and their event free survival with sorafenib ranged from 2 to 20 months. All four patients who accepted stem cell transplantation were still alive at the end of follow-up no matter they responded to sorafinib or not. The median survival time initiating from sorafenib usage has been 650 days.

Conclusion
The preliminary study on single sorafenib treatment responses of R/R AML with high CD117 expression and no FLT3-ITD mutation gave an encouraging result. Sorafinib, at least as a bridge to transplantation, should be considered in such special R/R AML patients in further study.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, C-kit, Flt3-ITD, Refractory


