
Contributions
Abstract: PB1746
Type: Publication Only
Background
Promyelocytic sarcoma (PS) following renal transplantation is an extremely rare phenomenon. Here we report a 26-year-old male suffered with PS at the site of transplanted ureter six months after renal transplant. Bone marrow aspiration and biopsy were normal, but Bcr1 subtype (intron 6) of the PML/RARα rearrangement gene was showed positive with 0.005 through common myeloid leukemia associated mutation genes and fusion genes screening. Chromosome was 46, XY. Flow cytometry detection of bone marrow cells had no special found. Then, fluorescence in situ hybridization (FISH) for PML/RARα rearrangement was performed on the ureteral tumor and it suggested positive at 15q22/17q21.
Aims
As the lesion seemed to be limited in the transplanted tissue, we sought to determine whether the tumor was a transmission of cancer from organ donor to the recipient.
Methods
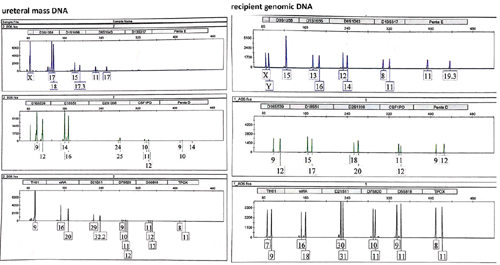
In order to address this hypothesis, DNA was extracted from the excised ureteral mass and compared with DNA from the patient’s blood. Just like as we suspected, DNA by short tandem repeat analysis revealed that ureteral mass was not of recipient origin.
The young donor was died from cerebral hemorrhage. We proposed that she may be suffered from disseminated intravascular coagulation, which is the severe and common complication of acute promyelocytic leukemia.
Results
Chemotherapy (arsenic trioxide 0.15mg/kg/d on d1–28, and ATRA 40mg/d for 28 days) was administered to induce remission. Immunosuppression therapy was changed into rapamycin single agent. One month later, PML/RARα fusion gene from bone marrow sample was negative. Thereafter he received arsenic trioxide five days a week with 0.15mg/kg/d for 4 weeks every 8 weeks, and ATRA 40mg/d on d1-14 every 28 days for 28 weeks as the post-remission therapy recommended by Lo-Coco F published in New England Journal of Medicin. This patient maintains complete remission for nearly 2 years. Now his renal function sustains on a normal level and the urinary tract is unobstructed.
Till now, the other recipient of this deceased donor’s kidney hasn’t caught by tumor.
Conclusion
Malignancy, as a major complication of renal transplant, accounts for 20% of the exitus of renal transplant patients every year and accounts for 30% of the death causes of the renal transplant recipients with a follow-up greater than 20 years. PS in the complete absence of bone marrow disease is an extremely rare phenomenon. To our knowledge, there had been only 2 patients presenting with promyelocytic sarcoma following renal transplant in the past three decades. They both suffered kidney failure again and subsequently had an allograft nephrectomy during the following chemotherapy.
Management of recipients with de novo cancers after transplantation is complex and difficult. Recommendations for cancer screening in the renal transplant population (including donors and recipients) were mostly extrapolated from the general population. It’s better to reduce the intensity of the combined chemotherapy and adjust immunosuppression therapy to protect the function of fragile allograft once de novo tumor occurred.
Session topic: 4. Acute myeloid leukemia - Clinical
Abstract: PB1746
Type: Publication Only
Background
Promyelocytic sarcoma (PS) following renal transplantation is an extremely rare phenomenon. Here we report a 26-year-old male suffered with PS at the site of transplanted ureter six months after renal transplant. Bone marrow aspiration and biopsy were normal, but Bcr1 subtype (intron 6) of the PML/RARα rearrangement gene was showed positive with 0.005 through common myeloid leukemia associated mutation genes and fusion genes screening. Chromosome was 46, XY. Flow cytometry detection of bone marrow cells had no special found. Then, fluorescence in situ hybridization (FISH) for PML/RARα rearrangement was performed on the ureteral tumor and it suggested positive at 15q22/17q21.
Aims
As the lesion seemed to be limited in the transplanted tissue, we sought to determine whether the tumor was a transmission of cancer from organ donor to the recipient.
Methods
In order to address this hypothesis, DNA was extracted from the excised ureteral mass and compared with DNA from the patient’s blood. Just like as we suspected, DNA by short tandem repeat analysis revealed that ureteral mass was not of recipient origin.
The young donor was died from cerebral hemorrhage. We proposed that she may be suffered from disseminated intravascular coagulation, which is the severe and common complication of acute promyelocytic leukemia.
Results
Chemotherapy (arsenic trioxide 0.15mg/kg/d on d1–28, and ATRA 40mg/d for 28 days) was administered to induce remission. Immunosuppression therapy was changed into rapamycin single agent. One month later, PML/RARα fusion gene from bone marrow sample was negative. Thereafter he received arsenic trioxide five days a week with 0.15mg/kg/d for 4 weeks every 8 weeks, and ATRA 40mg/d on d1-14 every 28 days for 28 weeks as the post-remission therapy recommended by Lo-Coco F published in New England Journal of Medicin. This patient maintains complete remission for nearly 2 years. Now his renal function sustains on a normal level and the urinary tract is unobstructed.
Till now, the other recipient of this deceased donor’s kidney hasn’t caught by tumor.

Conclusion
Malignancy, as a major complication of renal transplant, accounts for 20% of the exitus of renal transplant patients every year and accounts for 30% of the death causes of the renal transplant recipients with a follow-up greater than 20 years. PS in the complete absence of bone marrow disease is an extremely rare phenomenon. To our knowledge, there had been only 2 patients presenting with promyelocytic sarcoma following renal transplant in the past three decades. They both suffered kidney failure again and subsequently had an allograft nephrectomy during the following chemotherapy.
Management of recipients with de novo cancers after transplantation is complex and difficult. Recommendations for cancer screening in the renal transplant population (including donors and recipients) were mostly extrapolated from the general population. It’s better to reduce the intensity of the combined chemotherapy and adjust immunosuppression therapy to protect the function of fragile allograft once de novo tumor occurred.
Session topic: 4. Acute myeloid leukemia - Clinical


