
Contributions
Abstract: PB1743
Type: Publication Only
Background
In order to cure Acute Myeloid Leukemia (AML) intensive chemotherapy +/- Stem Cell Transplant (SCT) is require. Nevertheless, the median age of these patients is around 70 years. Elderly patients, defined in the AML literature as aged ≥60 years, historically have lower complete remission (CR) and relapse-free survival (RFS) rates than their younger counterparts and, in practice, only a minority of them can receive intensive treatments. For the majority of patients, the options are demethylating agents or supportive care.
Aims
To analyze the outcome of AML patients ≥ 60 years old according to the different therapeutic approach in a single institution. ts, defined in the AML literature as aged ≥60 years, historically have lower complete remission (CR) and relapse-free survival (RFS) rates than their younger counterparts and, in practice, only a minority of them can receive intensive treatments. For the majority of patients, the options are demethylating agents or supportive care.
Methods
Unicentric, retrospective analysis of AML patients ≥ 60 years old, not treated previously and diagnosed from 2011 to 2016. Statistical analysis was performed using SPSS v.15.0.
Results
Patients’ characteristics: Sixty-nine patients were registered: 51% male, median age 69 years old (range 60-90), 87% ECOG ≤ 1, 52% de novo and 48% secondary, and 22% with monosomal or complex karyotype. 49 patients (71%) received induction chemotherapy, 6 patients (9%) demethylating agents, only one patient (1%) received chemotherapy plus demethylating agents and 13 (19%) supportive care. Among patients treated with chemotherapy (n=49), 23 patients (47%) received consolidation with SCT (19 allogenic and 4 autologous) and 13 (26%) received demethylating maintenance. Survival: Median follow-up for alive patients was 42 months (range 4-81 months). Median overall survival (OS) from diagnosis according to the initial treatment was: 17 months (CI95% 10-23) for patients treated with chemotherapy (n= 49), 9 months for patients treated with demethylating agents (n=6) (CI95% 0-25), 8 months for the only patient who received chemotherapy plus demethylating agents and less than 1 month for those that received supportive care (n=13) (p<0.001). For patients treated in induction with chemotherapy, neither age (60-69 vs 70-79), type AML (de novo vs secondary), FLT-3 mutation, or SCT as consolidation (allogenic or autologous) have an impact in overall survival. Nevertheless, no complex/monosomic karyotype (p=0.01) and receiving demethylating maintenance (p=0.02) were associated with a better outcome. Patients treated in induction with chemotherapy plus maintenance with demethylating agents compared with those that received SCT, were older (p<0.001) but showed no differences in the main characteristics of their leukemia (secondary, karyotype and FLT3).
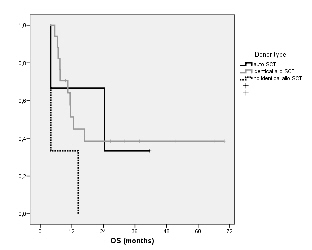
Median overall survival in transplanted patients (n=23) was as follows: 24 months for auto-SCT (n=3), 12 months in HLA-identical related/unrelated SCT (n=17) and 4 months in haploidentical or mismatched HLA unrelated SCT (n=3) (p=0.1). Both auto-SCT and identical allo-SCT observed plateau in survival graphics (Figure 1).
Conclusion
Our study shows the outcome of 69 older patients with AML in our current practical clinical. If patients are fit induction chemotherapy seems to be the best option to achieve longer outcomes but the post-induction isn’t clear and have to be personalised.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, Elderly
Abstract: PB1743
Type: Publication Only
Background
In order to cure Acute Myeloid Leukemia (AML) intensive chemotherapy +/- Stem Cell Transplant (SCT) is require. Nevertheless, the median age of these patients is around 70 years. Elderly patients, defined in the AML literature as aged ≥60 years, historically have lower complete remission (CR) and relapse-free survival (RFS) rates than their younger counterparts and, in practice, only a minority of them can receive intensive treatments. For the majority of patients, the options are demethylating agents or supportive care.
Aims
To analyze the outcome of AML patients ≥ 60 years old according to the different therapeutic approach in a single institution. ts, defined in the AML literature as aged ≥60 years, historically have lower complete remission (CR) and relapse-free survival (RFS) rates than their younger counterparts and, in practice, only a minority of them can receive intensive treatments. For the majority of patients, the options are demethylating agents or supportive care.
Methods
Unicentric, retrospective analysis of AML patients ≥ 60 years old, not treated previously and diagnosed from 2011 to 2016. Statistical analysis was performed using SPSS v.15.0.
Results
Patients’ characteristics: Sixty-nine patients were registered: 51% male, median age 69 years old (range 60-90), 87% ECOG ≤ 1, 52% de novo and 48% secondary, and 22% with monosomal or complex karyotype. 49 patients (71%) received induction chemotherapy, 6 patients (9%) demethylating agents, only one patient (1%) received chemotherapy plus demethylating agents and 13 (19%) supportive care. Among patients treated with chemotherapy (n=49), 23 patients (47%) received consolidation with SCT (19 allogenic and 4 autologous) and 13 (26%) received demethylating maintenance. Survival: Median follow-up for alive patients was 42 months (range 4-81 months). Median overall survival (OS) from diagnosis according to the initial treatment was: 17 months (CI95% 10-23) for patients treated with chemotherapy (n= 49), 9 months for patients treated with demethylating agents (n=6) (CI95% 0-25), 8 months for the only patient who received chemotherapy plus demethylating agents and less than 1 month for those that received supportive care (n=13) (p<0.001). For patients treated in induction with chemotherapy, neither age (60-69 vs 70-79), type AML (de novo vs secondary), FLT-3 mutation, or SCT as consolidation (allogenic or autologous) have an impact in overall survival. Nevertheless, no complex/monosomic karyotype (p=0.01) and receiving demethylating maintenance (p=0.02) were associated with a better outcome. Patients treated in induction with chemotherapy plus maintenance with demethylating agents compared with those that received SCT, were older (p<0.001) but showed no differences in the main characteristics of their leukemia (secondary, karyotype and FLT3).
Median overall survival in transplanted patients (n=23) was as follows: 24 months for auto-SCT (n=3), 12 months in HLA-identical related/unrelated SCT (n=17) and 4 months in haploidentical or mismatched HLA unrelated SCT (n=3) (p=0.1). Both auto-SCT and identical allo-SCT observed plateau in survival graphics (Figure 1).

Conclusion
Our study shows the outcome of 69 older patients with AML in our current practical clinical. If patients are fit induction chemotherapy seems to be the best option to achieve longer outcomes but the post-induction isn’t clear and have to be personalised.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, Elderly


