
Contributions
Abstract: PB1681
Type: Publication Only
Background
Acute myeloid leukemia (AML) is an aggressive hematopoietic malignancy characterized by diverse genetic and molecular abnormalities which contribute to poor response and survival rates. Spleen tyrosine kinase (SYK) signaling induces cell survival and proliferation by activating multiple pathways including chemokine regulation. Entospletinib (ENTO), an oral selective SYK inhibitor, is currently in clinical trials in AML. Previously we have shown that ENTO treatment inhibited BCR-mediated chemokines such as CCL3 and CCL4 in CLL patients (Sharman J et al., 2015). This retrospective longitudinal systemic biomarker analysis aims to understand the effects of SYK inhibition by ENTO during lead-in monotherapy and induction chemotherapy (IC) in untreated AML patients (NCT02343939).
Aims
(1) Explore the pharmacodynamic (PD) effects of ENTO during monotherapy lead-in on systemic biomarkers related to SYK and or AML disease (2) Correlate baseline biomarkers with complete remission (CR) rates and various molecularly defined AML sub-groups [NPM1, FLT3-ITD/TKD and KMT2A/mixed lineage leukemia gene rearrangements (MLL-R)].
Methods
Longitudinal plasma samples [baseline, 6hr post dose on day 1, day 8, and day 14 during ENTO monotherapy and post induction chemotherapy] from AML patients treated with ENTO monotherapy for up to 14 days and subsequently in combination with induction chemotherapy (7+3; cytarabine 100 mg/m2 for 7 days plus daunorubicin 60 mg/m2 for 3 days) were analyzed. A panel of 64 cytokines/chemokines including markers known to be regulated by SYK and key markers implicated in driving AML disease was tested. We utilized validated electrochemiluminescence multiplex immune assays from Meso Scale Discovery (MSD). Biomarker fold changes at each time point were compared to baseline using a Wilcoxon signed rank test. Correlations of these cytokines/chemokines levels at baseline with response rates and with various molecular subgroups were assessed using Wilcoxon rank-sum test.
Results
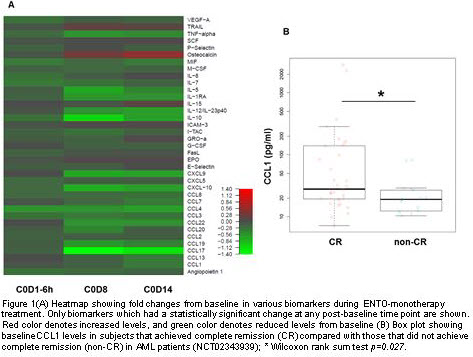
Chemokines such as CCL3, CCL4, CCL17 and CXCL-10 known to be regulated by SYK were significantly decreased from baseline after ENTO monotherapy. Additionally, key markers that have a role in driving AML disease such as IL-12p40, IL-1RA and TNFα were also significantly reduced. Heatmap of changes over the time in various biomarkers are presented in Figure 1A. Higher baseline CCL1 were observed in patients that achieved CR compared to non-CR (Figure 1 B; p=0.027). Further molecular sub-set analysis showed that patients with NPM1 mutation and with FLT3-ITD/TKD that were enriched in CR had high baseline CCL1 compared to wild type NPM1 and FLT3 patients. Baseline CCL1 levels were not significantly different between MLL-R patients and non-MLL-R patients even though most of MLL-R patients achieved CR suggesting underlying biology could be different in these sub-set of patients. Of note, in this study (NCT02343939) no ENTO exposure versus best overall response relationship was observed. Exploratory baseline versus post induction chemotherapy analysis is ongoing and updated results will be presented.
Conclusion
This is the first study that has demonstrated a PD effect of the SYK inhibitor Entospletinib during lead-in monotherapy in AML patients. In addition, baseline biomarker CCL-1 was observed to be associated with clinical response. Conclusions from this study are limited given this exploratory analysis in this small study and considering that no adjustments for multiple testing were applied to p-values, hence, all observations should be considered hypothesis generating.
Session topic: 3. Acute myeloid leukemia - Biology & Translational Research
Keyword(s): Acute Myeloid Leukemia, Chemokine, Cytokine, Tyrosine kinase
Abstract: PB1681
Type: Publication Only
Background
Acute myeloid leukemia (AML) is an aggressive hematopoietic malignancy characterized by diverse genetic and molecular abnormalities which contribute to poor response and survival rates. Spleen tyrosine kinase (SYK) signaling induces cell survival and proliferation by activating multiple pathways including chemokine regulation. Entospletinib (ENTO), an oral selective SYK inhibitor, is currently in clinical trials in AML. Previously we have shown that ENTO treatment inhibited BCR-mediated chemokines such as CCL3 and CCL4 in CLL patients (Sharman J et al., 2015). This retrospective longitudinal systemic biomarker analysis aims to understand the effects of SYK inhibition by ENTO during lead-in monotherapy and induction chemotherapy (IC) in untreated AML patients (NCT02343939).
Aims
(1) Explore the pharmacodynamic (PD) effects of ENTO during monotherapy lead-in on systemic biomarkers related to SYK and or AML disease (2) Correlate baseline biomarkers with complete remission (CR) rates and various molecularly defined AML sub-groups [NPM1, FLT3-ITD/TKD and KMT2A/mixed lineage leukemia gene rearrangements (MLL-R)].
Methods
Longitudinal plasma samples [baseline, 6hr post dose on day 1, day 8, and day 14 during ENTO monotherapy and post induction chemotherapy] from AML patients treated with ENTO monotherapy for up to 14 days and subsequently in combination with induction chemotherapy (7+3; cytarabine 100 mg/m2 for 7 days plus daunorubicin 60 mg/m2 for 3 days) were analyzed. A panel of 64 cytokines/chemokines including markers known to be regulated by SYK and key markers implicated in driving AML disease was tested. We utilized validated electrochemiluminescence multiplex immune assays from Meso Scale Discovery (MSD). Biomarker fold changes at each time point were compared to baseline using a Wilcoxon signed rank test. Correlations of these cytokines/chemokines levels at baseline with response rates and with various molecular subgroups were assessed using Wilcoxon rank-sum test.
Results
Chemokines such as CCL3, CCL4, CCL17 and CXCL-10 known to be regulated by SYK were significantly decreased from baseline after ENTO monotherapy. Additionally, key markers that have a role in driving AML disease such as IL-12p40, IL-1RA and TNFα were also significantly reduced. Heatmap of changes over the time in various biomarkers are presented in Figure 1A. Higher baseline CCL1 were observed in patients that achieved CR compared to non-CR (Figure 1 B; p=0.027). Further molecular sub-set analysis showed that patients with NPM1 mutation and with FLT3-ITD/TKD that were enriched in CR had high baseline CCL1 compared to wild type NPM1 and FLT3 patients. Baseline CCL1 levels were not significantly different between MLL-R patients and non-MLL-R patients even though most of MLL-R patients achieved CR suggesting underlying biology could be different in these sub-set of patients. Of note, in this study (NCT02343939) no ENTO exposure versus best overall response relationship was observed. Exploratory baseline versus post induction chemotherapy analysis is ongoing and updated results will be presented.

Conclusion
This is the first study that has demonstrated a PD effect of the SYK inhibitor Entospletinib during lead-in monotherapy in AML patients. In addition, baseline biomarker CCL-1 was observed to be associated with clinical response. Conclusions from this study are limited given this exploratory analysis in this small study and considering that no adjustments for multiple testing were applied to p-values, hence, all observations should be considered hypothesis generating.
Session topic: 3. Acute myeloid leukemia - Biology & Translational Research
Keyword(s): Acute Myeloid Leukemia, Chemokine, Cytokine, Tyrosine kinase


