
Contributions
Abstract: PB1680
Type: Publication Only
Background
Alteration of metabolic pathways induced by tumors may represent a potential target for therapeutic intervention. Acute myeloid leukemia (AML) mainly affects elderly subjects unfit for intensive chemotherapy in most cases. While AMLs have been extensively characterized in terms of genetic profile, little is known about their metabolic profile. Several agents currently tested in clinical trials interfere with metabolic pathways, including ascorbic acid (ASC), which is a potent pro-oxidant when used at high doses, and the anti-diabetic drug Buformin®.
Aims
To characterize metabolic pathways in different AML subsets and to assess the efficacy of Buformin® in combination with ASC in these tumors.
Methods
Using RQ-PCR and western Blot we analyzed in primary blasts from 50 AML patiens the expression levels of 7 metabolic enzymes: HK1, HK2, PKM2 and LDH, active in glycolysis control; PDH and PDK, involved in the synthesis of acetyl-CoA; and the regulators of fatty acid oxidation (FAO) CPTA1 and CT2. We measured oxygen consumption (OCR) and media acidification (ECAR) in cell cultures, by using the Seahorse XF Analyzer. We then studied the effects of metabolic-interference treating with Buformin® and ASC in different tumor cell lines: PR9 which derive from U937 cells and are PML-RARA-inducible, U937 transfected with the RUNX1-RUNX1T1 oncogene, OciAML3 AML2 cells, all AMLs and U87MG cells (glioblastoma). Buformin® was tested at 100 μM and 500 μM and ASC at 1mM concentrations. Apoptosis was assessed by flow cytometry using annexin and propidium-iodide staining.
Results
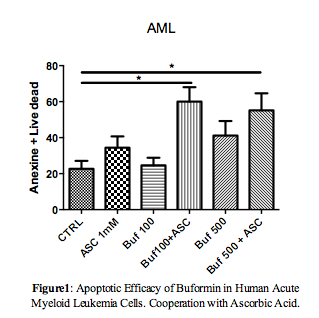
In patients with AML overexpression of PDH and CT2 mRNA was detected in primary Acute Promyelocytic Leukemia (APL) cells, as compared to other AML subtypes. CT2 overexpression was confirmed at the protein level and in PR9 cells. Concerning the metabolic activity, we demonstrated a decrease in glycolysis and a clear increase of mitochondrial respiration in the presence of PML-RARA, concomitant with the increase of PDH levels. In addition, increased levels of FAO were observed in association with CT2 overexpression. Conversely, expression of the RUNX1-RUNX1T1 transcript in inducible systems resulted in induction of glycolysis and mitochondrial respiration. In the presence of PML/RARA, Buformin® blocked the mitochondrial respiration and the consequent production of ATP in the Krebs cycle. ASC, alone or in combination with Buformin®, produced a slight increase in the cellular glycolytic capacity. Conversely, in RUNX1-RUNX1T1-positive cells, Buformin® shut down respiration, while in combination with ASC markedly decreased cellular glycolytic capacity. Treatment of both cell lines with Buformin® and ASC, blocked FAO, indicating that the cytotoxic effect could be partly due to the depletion of energy content. The pro-apoptotic effect was confirmed in primary blasts from seven AML patients by flow cytometry (p = 0.01, Figure 1). The series also included one case characterized by the rare PLZF-RARa transcript, which is ATRA and ATO resistant.
Conclusion
Our data provide insights on metabolic changes in AML subtypes and indicates the possibility of a metabolically-oriented therapy in these diseases. Although further studies are required to confirm these observations, our data suggests that the Buformin®-ASC combination could be tested as an innovative and cost-effective therapeutic option in AML patients unfit for intensive chemotherapy.
Session topic: 3. Acute myeloid leukemia - Biology & Translational Research
Keyword(s): AML1-ETO, Mitochondria, PML-RAR, Therapy
Abstract: PB1680
Type: Publication Only
Background
Alteration of metabolic pathways induced by tumors may represent a potential target for therapeutic intervention. Acute myeloid leukemia (AML) mainly affects elderly subjects unfit for intensive chemotherapy in most cases. While AMLs have been extensively characterized in terms of genetic profile, little is known about their metabolic profile. Several agents currently tested in clinical trials interfere with metabolic pathways, including ascorbic acid (ASC), which is a potent pro-oxidant when used at high doses, and the anti-diabetic drug Buformin®.
Aims
To characterize metabolic pathways in different AML subsets and to assess the efficacy of Buformin® in combination with ASC in these tumors.
Methods
Using RQ-PCR and western Blot we analyzed in primary blasts from 50 AML patiens the expression levels of 7 metabolic enzymes: HK1, HK2, PKM2 and LDH, active in glycolysis control; PDH and PDK, involved in the synthesis of acetyl-CoA; and the regulators of fatty acid oxidation (FAO) CPTA1 and CT2. We measured oxygen consumption (OCR) and media acidification (ECAR) in cell cultures, by using the Seahorse XF Analyzer. We then studied the effects of metabolic-interference treating with Buformin® and ASC in different tumor cell lines: PR9 which derive from U937 cells and are PML-RARA-inducible, U937 transfected with the RUNX1-RUNX1T1 oncogene, OciAML3 AML2 cells, all AMLs and U87MG cells (glioblastoma). Buformin® was tested at 100 μM and 500 μM and ASC at 1mM concentrations. Apoptosis was assessed by flow cytometry using annexin and propidium-iodide staining.
Results
In patients with AML overexpression of PDH and CT2 mRNA was detected in primary Acute Promyelocytic Leukemia (APL) cells, as compared to other AML subtypes. CT2 overexpression was confirmed at the protein level and in PR9 cells. Concerning the metabolic activity, we demonstrated a decrease in glycolysis and a clear increase of mitochondrial respiration in the presence of PML-RARA, concomitant with the increase of PDH levels. In addition, increased levels of FAO were observed in association with CT2 overexpression. Conversely, expression of the RUNX1-RUNX1T1 transcript in inducible systems resulted in induction of glycolysis and mitochondrial respiration. In the presence of PML/RARA, Buformin® blocked the mitochondrial respiration and the consequent production of ATP in the Krebs cycle. ASC, alone or in combination with Buformin®, produced a slight increase in the cellular glycolytic capacity. Conversely, in RUNX1-RUNX1T1-positive cells, Buformin® shut down respiration, while in combination with ASC markedly decreased cellular glycolytic capacity. Treatment of both cell lines with Buformin® and ASC, blocked FAO, indicating that the cytotoxic effect could be partly due to the depletion of energy content. The pro-apoptotic effect was confirmed in primary blasts from seven AML patients by flow cytometry (p = 0.01, Figure 1). The series also included one case characterized by the rare PLZF-RARa transcript, which is ATRA and ATO resistant.

Conclusion
Our data provide insights on metabolic changes in AML subtypes and indicates the possibility of a metabolically-oriented therapy in these diseases. Although further studies are required to confirm these observations, our data suggests that the Buformin®-ASC combination could be tested as an innovative and cost-effective therapeutic option in AML patients unfit for intensive chemotherapy.
Session topic: 3. Acute myeloid leukemia - Biology & Translational Research
Keyword(s): AML1-ETO, Mitochondria, PML-RAR, Therapy


