
Contributions
Abstract: PB1702
Type: Publication Only
Background
Increased intrablastic S100A8 has been associated with poor prognosis in AML. Moreover, its extracellular damage-associated molecular pattern activity regulates progression through genotoxic stress and myeloid differentiation independently of its frequently co-expressed protein S100A9. But extracellular S100A8 expression is not completely understood in AML.
Aims
In this context, we hypothesized that S100A8 can be secreted into BM niche and probably influences leukemic cell behavior and characterized its presence in AML.
Methods
Thus, we measured the level of S100A8 ([S100A8]) in 71 bone marrow plasma including 50 de novo AML, 12 myeloproliferative neoplasms (MPN), 7 myelodysplastic syndromes (MDS) and 9 healthy donors by ELISA technique. All patients gave their informed consent.
Results
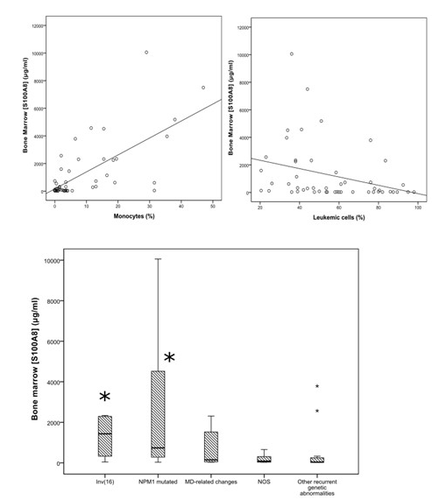
Firstly, we found AML patients had significantly higher rates than healthy subjects but also than MDS or MPN patients. A significant correlation between bone marrow and blood plasma existed but bone marrow [S100A8] was 7 times higher than blood levels. We found a direct link between [S100A8] and the intensity of cell proliferation (peripheral and medullar leukocytosis). The linear correlation between [S100A8] and the monocyte count suggests that monocytes could be the main producers in the bone marrow microenvironment. No correlation was observed between [S100A8] and blasts nor with the neutrophil count. This hypothesis was reinforced by the particularly high rates of S100A8 observed in AML4 and AML5 of the French American British classification. S100A8 levels were significantly higher in AML with molecular abnormalities such as mutated NPM1 or inv (16) of favorable prognosis. However, in the overall population, [S100A8] did not impact on remission rate, overall survival, or relapse rate. The cutpoint of 300µg/ml corresponding to the mean of S100A8 levels in AML was used to divide high and low expression of S100A8 in BM. The impact on prognosis was observed among mutated NPM1 and FLT3-ITD population for whom high levels of S100A8 were associated with significantly higher survival, compared to low S100A8 releasers.
Conclusion
In total, our study shows that there is a secretion of S100A8, probably of monocytic inflammatory origin, depending on the molecular status of patients that contributes to their prognosis.
Session topic: 3. Acute myeloid leukemia - Biology & Translational Research
Keyword(s): acute leukemia, Microenvironment
Abstract: PB1702
Type: Publication Only
Background
Increased intrablastic S100A8 has been associated with poor prognosis in AML. Moreover, its extracellular damage-associated molecular pattern activity regulates progression through genotoxic stress and myeloid differentiation independently of its frequently co-expressed protein S100A9. But extracellular S100A8 expression is not completely understood in AML.
Aims
In this context, we hypothesized that S100A8 can be secreted into BM niche and probably influences leukemic cell behavior and characterized its presence in AML.
Methods
Thus, we measured the level of S100A8 ([S100A8]) in 71 bone marrow plasma including 50 de novo AML, 12 myeloproliferative neoplasms (MPN), 7 myelodysplastic syndromes (MDS) and 9 healthy donors by ELISA technique. All patients gave their informed consent.
Results
Firstly, we found AML patients had significantly higher rates than healthy subjects but also than MDS or MPN patients. A significant correlation between bone marrow and blood plasma existed but bone marrow [S100A8] was 7 times higher than blood levels. We found a direct link between [S100A8] and the intensity of cell proliferation (peripheral and medullar leukocytosis). The linear correlation between [S100A8] and the monocyte count suggests that monocytes could be the main producers in the bone marrow microenvironment. No correlation was observed between [S100A8] and blasts nor with the neutrophil count. This hypothesis was reinforced by the particularly high rates of S100A8 observed in AML4 and AML5 of the French American British classification. S100A8 levels were significantly higher in AML with molecular abnormalities such as mutated NPM1 or inv (16) of favorable prognosis. However, in the overall population, [S100A8] did not impact on remission rate, overall survival, or relapse rate. The cutpoint of 300µg/ml corresponding to the mean of S100A8 levels in AML was used to divide high and low expression of S100A8 in BM. The impact on prognosis was observed among mutated NPM1 and FLT3-ITD population for whom high levels of S100A8 were associated with significantly higher survival, compared to low S100A8 releasers.

Conclusion
In total, our study shows that there is a secretion of S100A8, probably of monocytic inflammatory origin, depending on the molecular status of patients that contributes to their prognosis.
Session topic: 3. Acute myeloid leukemia - Biology & Translational Research
Keyword(s): acute leukemia, Microenvironment


