
Contributions
Abstract: PB1671
Type: Publication Only
Background
Na/H exchanger 1 (NHE1), an important participant in the precise regulation system of intracellular pH (pHi), is known to be involved in pathological processes of the neoplastic disease.
Aims
In this study, we evaluated the intracellular (pHi) and NHE1 expression in acute myeloid leukemia (AML) cell lines, and whether it contributes the resistance to cytarabine (AraC).
Methods
We evaluated the pHi and NHE1 expression in AML cell lines AraC sensitive OCI-AML2 (AS-OCI) and AraC resistant OCI-AML2 (AR-OCI) cells. To modulate the NHE1 activity, the NHE1 inhibitor, 5-(N, N-hexamethylene) amiloride (HMA), and NHE1 activator, phorbol 12-myristate 13-acetate (PMA), were used. The level of apoptosis and proliferation induced by NHE1 inhibitor or activator with/without AraC was assessed at 24 hr after treatment by the annexin V assays and the CCK-8 assay.
Results
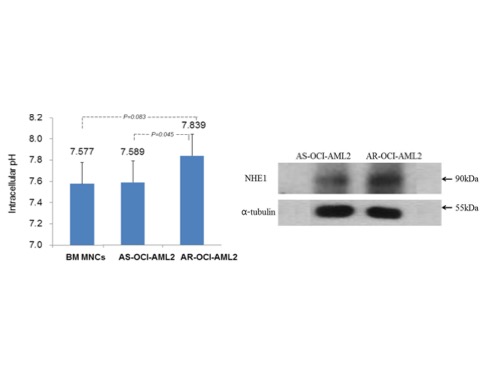
The pHi of AR-OCI cells was significantly higher than AS-OCI cells (7.839 vs 7.589, P=.045). Compared with AS-OCI cells, AR-OCI showed significantly higher NHE1 expression by western blot analysis, and NHE1 mRNA levels (1.565 vs 0.039, P<.01) by qRT-PCR. After 24hr treatment with HMA 10 ~ 30 µM, the proliferation of leukemic cells was inhibited, and the apoptosis was induced in a concentration-dependent manner, and higher concentration of HMA (30 µM) could induced apoptosis on most of AR-OCI cells. When treated by PMA with various concentration form 0.1 to 10 uM, proliferation of leukemic cells was inhibited in a concentration-dependent manner, but apoptosis was not induced. The addition of HMA 10 µM that concentration did not cause apoptosis of AR-OCI cells when treated alone to AraC treatment resulted in a significant increase in the fraction of apoptosis in AR-OCI cells (P<0.001) as compared with that of AraC treatment alone (26.33% vs 7.11%, P<.001). Also, the addition of PMA 1 µM to AraC treatment resulted in a significant increase in the level of apoptosis in AR-OCI cells as compared with that of AraC treatment alone (45.70% vs 7.11%, P<.001). These findings suggest that NHE1 plays an important role in resistance mechanism to AraC in AML cell line and NHE1 might be potential therapeutic target to overcome chemoresistance in AML. To elucidate the detailed signaling pathway after modulation of NHE1 activity, further experimental studies have been proceeding.
Conclusion
These findings suggest that NHE1 plays an important role in resistance mechanism to AraC in AML cell line and NHE1 might be potential therapeutic target to overcome chemoresistance in AML. To elucidate the detailed signaling pathway after modulation of NHE1 activity, further experimental studies have been proceeding.
Session topic: 3. Acute myeloid leukemia - Biology & Translational Research
Keyword(s): AML, Cytarabine, Resistance
Abstract: PB1671
Type: Publication Only
Background
Na/H exchanger 1 (NHE1), an important participant in the precise regulation system of intracellular pH (pHi), is known to be involved in pathological processes of the neoplastic disease.
Aims
In this study, we evaluated the intracellular (pHi) and NHE1 expression in acute myeloid leukemia (AML) cell lines, and whether it contributes the resistance to cytarabine (AraC).
Methods
We evaluated the pHi and NHE1 expression in AML cell lines AraC sensitive OCI-AML2 (AS-OCI) and AraC resistant OCI-AML2 (AR-OCI) cells. To modulate the NHE1 activity, the NHE1 inhibitor, 5-(N, N-hexamethylene) amiloride (HMA), and NHE1 activator, phorbol 12-myristate 13-acetate (PMA), were used. The level of apoptosis and proliferation induced by NHE1 inhibitor or activator with/without AraC was assessed at 24 hr after treatment by the annexin V assays and the CCK-8 assay.
Results
The pHi of AR-OCI cells was significantly higher than AS-OCI cells (7.839 vs 7.589, P=.045). Compared with AS-OCI cells, AR-OCI showed significantly higher NHE1 expression by western blot analysis, and NHE1 mRNA levels (1.565 vs 0.039, P<.01) by qRT-PCR. After 24hr treatment with HMA 10 ~ 30 µM, the proliferation of leukemic cells was inhibited, and the apoptosis was induced in a concentration-dependent manner, and higher concentration of HMA (30 µM) could induced apoptosis on most of AR-OCI cells. When treated by PMA with various concentration form 0.1 to 10 uM, proliferation of leukemic cells was inhibited in a concentration-dependent manner, but apoptosis was not induced. The addition of HMA 10 µM that concentration did not cause apoptosis of AR-OCI cells when treated alone to AraC treatment resulted in a significant increase in the fraction of apoptosis in AR-OCI cells (P<0.001) as compared with that of AraC treatment alone (26.33% vs 7.11%, P<.001). Also, the addition of PMA 1 µM to AraC treatment resulted in a significant increase in the level of apoptosis in AR-OCI cells as compared with that of AraC treatment alone (45.70% vs 7.11%, P<.001). These findings suggest that NHE1 plays an important role in resistance mechanism to AraC in AML cell line and NHE1 might be potential therapeutic target to overcome chemoresistance in AML. To elucidate the detailed signaling pathway after modulation of NHE1 activity, further experimental studies have been proceeding.

Conclusion
These findings suggest that NHE1 plays an important role in resistance mechanism to AraC in AML cell line and NHE1 might be potential therapeutic target to overcome chemoresistance in AML. To elucidate the detailed signaling pathway after modulation of NHE1 activity, further experimental studies have been proceeding.
Session topic: 3. Acute myeloid leukemia - Biology & Translational Research
Keyword(s): AML, Cytarabine, Resistance


