
Contributions
Abstract: PB1635
Type: Publication Only
Background
Minimal residual disease (MRD) is an important independent prognostic factor that can identify poor responders among patients with B-acute lymphoblastic leukemia (ALL). The fundamental principle of the detection of MRD by flow cytometry is that leukemic cells show altered patterns of antigen expression. There are ongoing efforts to standardize MRD quantification using flow cytometry to improve accuracy and reproducibility, because hematogones that may morphologically resemble the leukemic cells of B-ALL are often increased in regenerating marrow following chemotherapy or hematopoietic stem cell transplantation.
Aims
The aim of this study was to evaluate two commercial kits for flow cytometric MRD detection in B-ALL.
Methods
50 bone marrow aspirates with less than 5% leukemic cells by conventional morphology from treated 30 pediatric patients with B-ALL were obtained. MRD was measured using two commercial kits for flow cytometeric MRD detection; DuraClone RE ALB Tube (Beckman Coulter, Miami, USA) and BCP-ALL-MRD (Cytognos SL, Salamanca, Spain). The main methodological approach for detecting MRD is to detect antigen over or under expression of DuraClone RE ALB Tube (CD58/CD34/CD10/CD19/CD38/CD20/CD45) and aberrant expression of BCP-ALL-MRD (tube1: CD20/CD45/CD81/CD66c+CD123/CD34/CD19/CD10/CD38, tube2: CD20/CD45/CD81/CD73+CD304/CD34/CD19/CD10/CD38). Acquisition cell numbers of DuraClone RE ALB Tube and BCP-ALL-MRD were 2.0x106 and 5.0x106, respectively. Flow cytometric analysis was performed by manual serial gating according to our protocol. All specimens for MRD detection were negative for by real-time quantitative polymerase chain reaction (RQ-PCR) or fluorescence in situ hybridization (FISH) according to genetic abnormalities at diagnosis except 4 bone marrow aspirates (minor BCR-ABL1 RQ-PCR: positive, normalized copy number: 13.41, 1.25, and 0.25 and % cells with MLL deletion, RUNX1 gain, and IGH gain by FISH: 0.50-0.75%).
Results
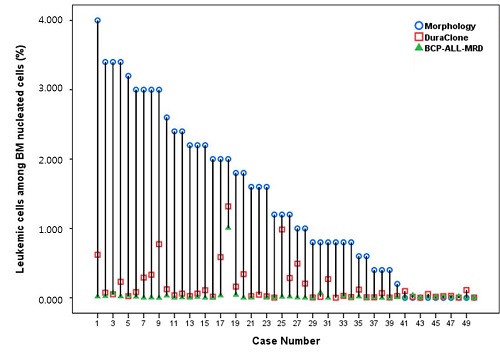
The median patient age was 7 years (range, 3-20) and the ratio of boy to girl was 1:1. The median time of sample collection was 153.5 days (14-3930) after diagnosis or relapse. The immunophenotype of leukemic cells at diagnosis in all patients showed CD10 positive (cutoff ≥20%) and variable CD34 and CD20 expression. Leukemic cells at diagnosis were all positive for CD58 but variable CD38 expression from negative to bright positive. Aberrant expression at diagnosis was CD66c (13/24, 54%), CD123 (12/24, 50%), CD73 (8/24, 33%), CD304 (1/24, 4%), CD13 (5/29, 17%), CD33 (3/29, 10%), and CD56 (1/29, 3%). The leukemic cell % (mean±SD) were 1.4±1.2 % by conventional morphology, 0.166±0.268 % by DuraClone RE ALB Tube and 0.034±0.143 % by BCP-ALL-MRD (Fig.1, P=0.001 in difference). MRD % by DuraClone RE ALB Tube and BCP-ALL-MRD were well correlated (P ≤0.001). However, the percentage of leukemic cells by conventional morphology show statistical significance with MRD % by DuraClone RE ALB Tube, but not with BCP-ALL-MRD (P=0.024 and P=0.521, respectively).
Conclusion
Detection of MRD in patients with B-ALL is limited by conventional morphology. For the flow cytometric detection of MRD in B-ALL, the kit detecting antigen over or under expression was adequate, but the kit detecting aberrant expression showed significantly lower level of MRD. It is very helpful for analysis of MRD to know the immunophenotype of leukemic cells at diagnosis.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Acute lymphoblastic leukemia, flow cytometry, Minimal residual disease (MRD)
Abstract: PB1635
Type: Publication Only
Background
Minimal residual disease (MRD) is an important independent prognostic factor that can identify poor responders among patients with B-acute lymphoblastic leukemia (ALL). The fundamental principle of the detection of MRD by flow cytometry is that leukemic cells show altered patterns of antigen expression. There are ongoing efforts to standardize MRD quantification using flow cytometry to improve accuracy and reproducibility, because hematogones that may morphologically resemble the leukemic cells of B-ALL are often increased in regenerating marrow following chemotherapy or hematopoietic stem cell transplantation.
Aims
The aim of this study was to evaluate two commercial kits for flow cytometric MRD detection in B-ALL.
Methods
50 bone marrow aspirates with less than 5% leukemic cells by conventional morphology from treated 30 pediatric patients with B-ALL were obtained. MRD was measured using two commercial kits for flow cytometeric MRD detection; DuraClone RE ALB Tube (Beckman Coulter, Miami, USA) and BCP-ALL-MRD (Cytognos SL, Salamanca, Spain). The main methodological approach for detecting MRD is to detect antigen over or under expression of DuraClone RE ALB Tube (CD58/CD34/CD10/CD19/CD38/CD20/CD45) and aberrant expression of BCP-ALL-MRD (tube1: CD20/CD45/CD81/CD66c+CD123/CD34/CD19/CD10/CD38, tube2: CD20/CD45/CD81/CD73+CD304/CD34/CD19/CD10/CD38). Acquisition cell numbers of DuraClone RE ALB Tube and BCP-ALL-MRD were 2.0x106 and 5.0x106, respectively. Flow cytometric analysis was performed by manual serial gating according to our protocol. All specimens for MRD detection were negative for by real-time quantitative polymerase chain reaction (RQ-PCR) or fluorescence in situ hybridization (FISH) according to genetic abnormalities at diagnosis except 4 bone marrow aspirates (minor BCR-ABL1 RQ-PCR: positive, normalized copy number: 13.41, 1.25, and 0.25 and % cells with MLL deletion, RUNX1 gain, and IGH gain by FISH: 0.50-0.75%).
Results
The median patient age was 7 years (range, 3-20) and the ratio of boy to girl was 1:1. The median time of sample collection was 153.5 days (14-3930) after diagnosis or relapse. The immunophenotype of leukemic cells at diagnosis in all patients showed CD10 positive (cutoff ≥20%) and variable CD34 and CD20 expression. Leukemic cells at diagnosis were all positive for CD58 but variable CD38 expression from negative to bright positive. Aberrant expression at diagnosis was CD66c (13/24, 54%), CD123 (12/24, 50%), CD73 (8/24, 33%), CD304 (1/24, 4%), CD13 (5/29, 17%), CD33 (3/29, 10%), and CD56 (1/29, 3%). The leukemic cell % (mean±SD) were 1.4±1.2 % by conventional morphology, 0.166±0.268 % by DuraClone RE ALB Tube and 0.034±0.143 % by BCP-ALL-MRD (Fig.1, P=0.001 in difference). MRD % by DuraClone RE ALB Tube and BCP-ALL-MRD were well correlated (P ≤0.001). However, the percentage of leukemic cells by conventional morphology show statistical significance with MRD % by DuraClone RE ALB Tube, but not with BCP-ALL-MRD (P=0.024 and P=0.521, respectively).

Conclusion
Detection of MRD in patients with B-ALL is limited by conventional morphology. For the flow cytometric detection of MRD in B-ALL, the kit detecting antigen over or under expression was adequate, but the kit detecting aberrant expression showed significantly lower level of MRD. It is very helpful for analysis of MRD to know the immunophenotype of leukemic cells at diagnosis.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Acute lymphoblastic leukemia, flow cytometry, Minimal residual disease (MRD)


