
Contributions
Abstract: PB1634
Type: Publication Only
Background
Asparaginase is an essential drug for treatment of pediatric acute lymphoblastic leukemia (ALL). It acts by enzymatic cleavage of L-asparagine amino acid into aspartic acid and ammonia, thus depriving the leukemic cells from L-asparagine, resulting into cell death. Three preparations are available; the native asparaginase derived from Escherichia coli (E. coli-asparaginase), a pegylated form (PEG-asparaginase) and a product isolated from Erwinia chrysanthemi, i.e. Erwinia asparaginase. Owing to its easier administration and less frequent injections, our patients receive PEG-asparaginase as a standard of care.
Aims
To review the clinical presentations, radiological characteristics, management and prognosis of asparaginase-related adverse reactions in Omani children with ALL.
Methods
A retrospective cohort study of all children diagnosed with ALL who developed asparaginase-induced adverse effects while being on chemotherapy during the period January 2006 through January 2018.
Results
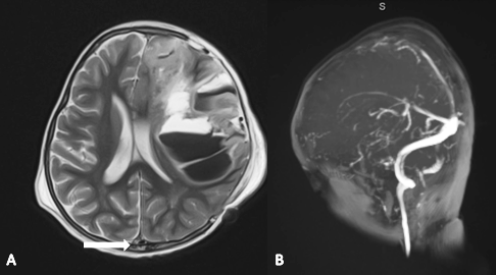
Out of 198 pediatric patients with ALL, 11 children (8 males and 3 females) developed severe asparaginase-related adverse effects, constituting 5.6% of the total number of patients. Severe full blown pancreatitis was encountered in only one patient (0.05%). He is an 11 year-old male which has survived this stormy mishap, but developed permanent diabetes mellitus that required lifelong insulin therapy. He has never been exposed to any further doses of asparaginase, and then went into complete remission. Two patients developed severe anaphylactic reaction, and then shifted to erwinia asparaginase with no recurrence. Notably, eight of our patients developed asparaginase-related cerebral venous sinus thrombosis, evidenced by contrast-enhanced cerebral MRI. The mean duration of stroke diagnosis was (6±2.1) days after the last asparaginase injection. All were managed conservatively with anticoagulation using unfractionated and/or low molecular weight heparin. Two of these patients have had a recurrence upon re-challenge, despite concomitant prophylactic heparin. Remarkably, seven patients (7/8) survived without any neurological sequelae. Unfortunately, a 30 month-old boy developed permanent right-sided hemiplegia secondary to extensive superior sagittal sinus thrombosis, which has been uniquely associated with massive left-sided fronto-parietal hemorrhage and midline shift (Figure). This patient required ICU admission and underwent a pressure-relieving craniectomy surgey. It has been planned not to expose him to any further asparaginase doses.
In addition to these severe reactions, 23 patients developed mild to moderate asparaginase-related side effects in the form of transient hyperglycemia that required insulin therapy in 17 patients, local skin reaction at the site of injection in 3 patients and transient urticarial skin rash in 3 patients as well.
Conclusion
Most of asparaginase-induced adverse reactions are transient and self-limited. However, a high index of suspicion is needed for early detection of intracranial thrombotic complications. Contrast-enhanced magnetic resonance venography (MRV), or CT venography are the modalities of choice. Early diagnosis, discontinuation of asparaginase and prompt initiation of anticoagulation are mandatory steps to prevent permanent neurological deficits.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Acute lymphoblastic leukemia, Asparaginase, Thrombosis
Abstract: PB1634
Type: Publication Only
Background
Asparaginase is an essential drug for treatment of pediatric acute lymphoblastic leukemia (ALL). It acts by enzymatic cleavage of L-asparagine amino acid into aspartic acid and ammonia, thus depriving the leukemic cells from L-asparagine, resulting into cell death. Three preparations are available; the native asparaginase derived from Escherichia coli (E. coli-asparaginase), a pegylated form (PEG-asparaginase) and a product isolated from Erwinia chrysanthemi, i.e. Erwinia asparaginase. Owing to its easier administration and less frequent injections, our patients receive PEG-asparaginase as a standard of care.
Aims
To review the clinical presentations, radiological characteristics, management and prognosis of asparaginase-related adverse reactions in Omani children with ALL.
Methods
A retrospective cohort study of all children diagnosed with ALL who developed asparaginase-induced adverse effects while being on chemotherapy during the period January 2006 through January 2018.
Results
Out of 198 pediatric patients with ALL, 11 children (8 males and 3 females) developed severe asparaginase-related adverse effects, constituting 5.6% of the total number of patients. Severe full blown pancreatitis was encountered in only one patient (0.05%). He is an 11 year-old male which has survived this stormy mishap, but developed permanent diabetes mellitus that required lifelong insulin therapy. He has never been exposed to any further doses of asparaginase, and then went into complete remission. Two patients developed severe anaphylactic reaction, and then shifted to erwinia asparaginase with no recurrence. Notably, eight of our patients developed asparaginase-related cerebral venous sinus thrombosis, evidenced by contrast-enhanced cerebral MRI. The mean duration of stroke diagnosis was (6±2.1) days after the last asparaginase injection. All were managed conservatively with anticoagulation using unfractionated and/or low molecular weight heparin. Two of these patients have had a recurrence upon re-challenge, despite concomitant prophylactic heparin. Remarkably, seven patients (7/8) survived without any neurological sequelae. Unfortunately, a 30 month-old boy developed permanent right-sided hemiplegia secondary to extensive superior sagittal sinus thrombosis, which has been uniquely associated with massive left-sided fronto-parietal hemorrhage and midline shift (Figure). This patient required ICU admission and underwent a pressure-relieving craniectomy surgey. It has been planned not to expose him to any further asparaginase doses.
In addition to these severe reactions, 23 patients developed mild to moderate asparaginase-related side effects in the form of transient hyperglycemia that required insulin therapy in 17 patients, local skin reaction at the site of injection in 3 patients and transient urticarial skin rash in 3 patients as well.

Conclusion
Most of asparaginase-induced adverse reactions are transient and self-limited. However, a high index of suspicion is needed for early detection of intracranial thrombotic complications. Contrast-enhanced magnetic resonance venography (MRV), or CT venography are the modalities of choice. Early diagnosis, discontinuation of asparaginase and prompt initiation of anticoagulation are mandatory steps to prevent permanent neurological deficits.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Acute lymphoblastic leukemia, Asparaginase, Thrombosis


