
Contributions
Abstract: PB1641
Type: Publication Only
Background
Blinatumomab is a bispecific T-cell antibody indicated for the treatment of adult and pediatric patients with Philadelphia chromosome-negative (Ph-) relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
Aims
We report 2 cases of response reach and maintenance to blinatumomab.
Methods
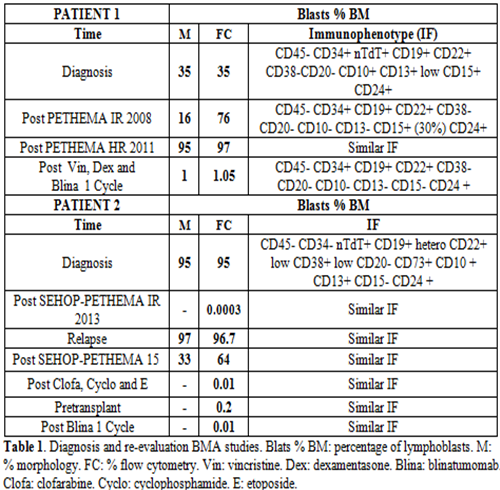
Case 1: A 21 years old man who presented peripheral blood (PB): leukocytes 2.4 x 10 3/µL, neutrophils 0.9 x 10 3/µL, hemoglobin 9.7 g/dL, platelets 22 x 10 3/µL. Lymphoblasts 1%. The bone marrow aspirate (BMA) with 35% of lymphoblasts. The immunophenotype (IF) (Table 1), cytogenetic and molecular biology analysis confirmed the diagnosis of B-II or common ALL Ph- (MLL negative). No central nervous system infiltration. He started induction therapy according to the PETHEMA ALL intermediate risk-2008 protocol with vincristine, daunorubicine and prednisone. BMA on day +14 without response, IF with 76% of blasts (Table 1), revealed diagnosis of more aggressive ALL: B-I or pro-B ALL Ph- (MLL negative). Due to it, he started reinduction of the PETHEMA ALL high risk-2011 protocol (Flag-IDA). Meantime an allogeneic stem cell transplant (ASCT) of unrelated donor was proposed. He presented as complications pulmonary fungal infection. On day +34 it showed lack of response, IF similar to the previous one (Table 1). A third line of treatment is proposed, starting blinatumomab after debulking with vincristine and dexamentasone. A Cytokine Release Syndrome (CRS) was reported after 3 weeks of treatment, therefore blinatumomab was delayed until CRS was controlled with conventional treatment. Reevaluation after the first cycle of blinatumomab, PB: leukocytes 2.7 x 10 3/µL, neutrophils 1.5 x 10 3/µL, hemoglobin 8,7 g/dL, platelets 41 x 10 3/µL and BMA showed morphological complete remission (CR) and minimal residual disease (MRD) + (Table 1). Finally, the patient died due to a new episode of pulmonary infection.
Case 2: A 15 years old man, PB: leukocytes 7.8 x 10 3/µL, neutrophils 1.2 x 10 3/µL, hemoglobin 8.4 g/dL, platelets 70 x 10 3/µL, lymphoblast: 53%. BMA: 95% of lymphoblast. The IF (Table 1), cytogenetic and molecular biology analysis of the BMA confirmed the diagnosis of B-II or common ALL Ph-, t (1;19) positive. TCF3 positive and MLL negative. No central nervous system infiltration. He started treatment according to the SEHOP-PETHEMA ALL 2013 intermediate risk protocol. Early relapsed with 97% of lymphoblast in BMA. He was treated under the therapeutic recommendations for relapse SEHOP-PETHEMA ALL 2015 without response, with 33% infiltration. Rescue chemotherapy with clofarabine, cyclophosphamide and etoposide, obtaining CR with MRD: 0.01% (Table 1). As complications, febrile neutropenia with bacteremia secondary to E. coli and aspergillosis with orbital, paranasal sinus and pulmonary affectation. Due to the delay of the transplant by infections and MRD: 0.2%, blinatumomab was initiated as a bridging therapy to transplant, being well tolerated. He maintained MRD lower than 0.01% after the first cycle (Table 1). An ASCT of identical HLA brother was performed. Currently in remission and complete chimerism.
Results
The results are shown in Table 1.
Conclusion
According to the published studies, patients with a low tumor load (<50% infiltration) obtain better results than patient with highest tumor (>90%); with CR rate of 72,9% vs. 21,6%. In the first case, a spectacular response was observed after treatment with blinatumomab with previous debulking therapy. This strategy can be an option for patients with high tumor burden.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Acute lymphoblastic leukemia, chemotherapy, Immune therapy
Abstract: PB1641
Type: Publication Only
Background
Blinatumomab is a bispecific T-cell antibody indicated for the treatment of adult and pediatric patients with Philadelphia chromosome-negative (Ph-) relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
Aims
We report 2 cases of response reach and maintenance to blinatumomab.
Methods
Case 1: A 21 years old man who presented peripheral blood (PB): leukocytes 2.4 x 10 3/µL, neutrophils 0.9 x 10 3/µL, hemoglobin 9.7 g/dL, platelets 22 x 10 3/µL. Lymphoblasts 1%. The bone marrow aspirate (BMA) with 35% of lymphoblasts. The immunophenotype (IF) (Table 1), cytogenetic and molecular biology analysis confirmed the diagnosis of B-II or common ALL Ph- (MLL negative). No central nervous system infiltration. He started induction therapy according to the PETHEMA ALL intermediate risk-2008 protocol with vincristine, daunorubicine and prednisone. BMA on day +14 without response, IF with 76% of blasts (Table 1), revealed diagnosis of more aggressive ALL: B-I or pro-B ALL Ph- (MLL negative). Due to it, he started reinduction of the PETHEMA ALL high risk-2011 protocol (Flag-IDA). Meantime an allogeneic stem cell transplant (ASCT) of unrelated donor was proposed. He presented as complications pulmonary fungal infection. On day +34 it showed lack of response, IF similar to the previous one (Table 1). A third line of treatment is proposed, starting blinatumomab after debulking with vincristine and dexamentasone. A Cytokine Release Syndrome (CRS) was reported after 3 weeks of treatment, therefore blinatumomab was delayed until CRS was controlled with conventional treatment. Reevaluation after the first cycle of blinatumomab, PB: leukocytes 2.7 x 10 3/µL, neutrophils 1.5 x 10 3/µL, hemoglobin 8,7 g/dL, platelets 41 x 10 3/µL and BMA showed morphological complete remission (CR) and minimal residual disease (MRD) + (Table 1). Finally, the patient died due to a new episode of pulmonary infection.
Case 2: A 15 years old man, PB: leukocytes 7.8 x 10 3/µL, neutrophils 1.2 x 10 3/µL, hemoglobin 8.4 g/dL, platelets 70 x 10 3/µL, lymphoblast: 53%. BMA: 95% of lymphoblast. The IF (Table 1), cytogenetic and molecular biology analysis of the BMA confirmed the diagnosis of B-II or common ALL Ph-, t (1;19) positive. TCF3 positive and MLL negative. No central nervous system infiltration. He started treatment according to the SEHOP-PETHEMA ALL 2013 intermediate risk protocol. Early relapsed with 97% of lymphoblast in BMA. He was treated under the therapeutic recommendations for relapse SEHOP-PETHEMA ALL 2015 without response, with 33% infiltration. Rescue chemotherapy with clofarabine, cyclophosphamide and etoposide, obtaining CR with MRD: 0.01% (Table 1). As complications, febrile neutropenia with bacteremia secondary to E. coli and aspergillosis with orbital, paranasal sinus and pulmonary affectation. Due to the delay of the transplant by infections and MRD: 0.2%, blinatumomab was initiated as a bridging therapy to transplant, being well tolerated. He maintained MRD lower than 0.01% after the first cycle (Table 1). An ASCT of identical HLA brother was performed. Currently in remission and complete chimerism.
Results
The results are shown in Table 1.

Conclusion
According to the published studies, patients with a low tumor load (<50% infiltration) obtain better results than patient with highest tumor (>90%); with CR rate of 72,9% vs. 21,6%. In the first case, a spectacular response was observed after treatment with blinatumomab with previous debulking therapy. This strategy can be an option for patients with high tumor burden.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Acute lymphoblastic leukemia, chemotherapy, Immune therapy


