
Contributions
Abstract: PB1616
Type: Publication Only
Background
NOTCH signaling is crucial for the growth of T-lymphoblastic leukemia (T-ALL) cells. We have previously reported that treatment with γ-secretase inhibitors (GSIs), which block NOTCH activation, suppresses the growth of several T-ALL cell lines. However, while GSI-XXI inhibited the proliferation of KOPT-K1 cellspromoted that of Jurkat cells, although both of these T-ALL cell lines harbor NOTCH1-activating mutations.
Aims
To elucidate the mechanism underlying the promotion of Jurkat cell growth by NOTCH inhibition.
Methods
KOPT-K1 and Jurkat cells were transfected with small interfering RNA (siRNA) targeting NOTCH1 (siN1) or NOTCH2 (siN2), or control siRNA (siCont) using the pipette tip chamber-based electroporation system. The effects of NOTCH knockdown on mRNA and protein expression were examined by quantitative RT-PCR and immunoblotting, respectively. Cell growth was assessed in three-day cultures by a colorimetric WST-8 assay, and the relative cell number was determined based on optical density measured using an ELISA plate reader and expressed as the percentage of the mean absorbance value normalized to that of siCont-transfected cells.
Results
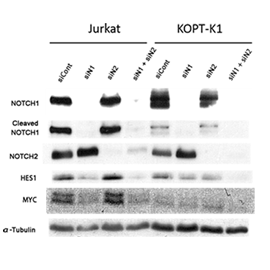
Transfection with siN1 and siN2 selectively inhibited the expression of NOTCH1 and NOTCH2 (mRNA and protein), respectively, both in KOPT-K1 and Jurkat cells. NOTCH1 and NOTCH2 knockdown suppressed the growth of KOPT-K1 cells to 60% and 67%, respectively, but promoted that of Jurkat cells to 158% and 152%, respectively, of control. In Jurkat cells, NOTCH1 knockdown abolished the expression of cleaved NOTCH1 fragment (active form of NOTCH1), downregulated HES1 and MYC, but slightly increased NOTCH2 levels, whereas NOTCH2 knockdown slightly upregulated the levels of cleaved NOTCH1 fragment, HES1, MYC, and NOTCH1. In KOPT-K1 cells, NOTCH1 knockdown decreased the expression of cleaved NOTCH1 fragment and HES1, while slightly increasing that of NOTCH2, whereas NOTCH2 knockdown did not affect the expression of other proteins. The knockdown of NOTCH1 and 2 did not affect the level of mTOR, Hedgehog, and Wnt signaling-related proteins in both cell lines.
Conclusion
In this study, we confirmed our previous findings that GSI-XXI treatment suppressed the growth of KOPT-K1 cells while promoting that of Jurkat cells using NOTCH1- and NOTCH2-specific siRNA. It is well known that NOTCH activation is oncogenic and promotes the growth of T-ALL cells. However, in Jurkat cells NOTCH obviously exerted the opposite effect. Interestingly, the knockdown of NOTCH2, similar to that of NOTCH1, affected the growth of the two cell lines, although they do not have NOTCH2-activating mutations. Furthermore, in Jurkat cells, we observed an interesting phenomenon that NOTCH1 knockdown upregulated NOTCH2 and vice versa, which might be related to the cell proliferation due to NOTCH suppression although this mechanism can not fully answer the question in the aim of this study. The underlying molecular mechanisms and the biological significance of the effect need to be further elucidated. Our results contribute to understanding of the role of NOTCH signaling in T-ALL cells and should promote the development of novel NOTCH-targeting therapies against T-ALL.
Session topic: 1. Acute lymphoblastic leukemia – Biology & Translational Research
Keyword(s): Notch, SiRNA, T cell acute lymphoblastic leukemia
Abstract: PB1616
Type: Publication Only
Background
NOTCH signaling is crucial for the growth of T-lymphoblastic leukemia (T-ALL) cells. We have previously reported that treatment with γ-secretase inhibitors (GSIs), which block NOTCH activation, suppresses the growth of several T-ALL cell lines. However, while GSI-XXI inhibited the proliferation of KOPT-K1 cellspromoted that of Jurkat cells, although both of these T-ALL cell lines harbor NOTCH1-activating mutations.
Aims
To elucidate the mechanism underlying the promotion of Jurkat cell growth by NOTCH inhibition.
Methods
KOPT-K1 and Jurkat cells were transfected with small interfering RNA (siRNA) targeting NOTCH1 (siN1) or NOTCH2 (siN2), or control siRNA (siCont) using the pipette tip chamber-based electroporation system. The effects of NOTCH knockdown on mRNA and protein expression were examined by quantitative RT-PCR and immunoblotting, respectively. Cell growth was assessed in three-day cultures by a colorimetric WST-8 assay, and the relative cell number was determined based on optical density measured using an ELISA plate reader and expressed as the percentage of the mean absorbance value normalized to that of siCont-transfected cells.
Results
Transfection with siN1 and siN2 selectively inhibited the expression of NOTCH1 and NOTCH2 (mRNA and protein), respectively, both in KOPT-K1 and Jurkat cells. NOTCH1 and NOTCH2 knockdown suppressed the growth of KOPT-K1 cells to 60% and 67%, respectively, but promoted that of Jurkat cells to 158% and 152%, respectively, of control. In Jurkat cells, NOTCH1 knockdown abolished the expression of cleaved NOTCH1 fragment (active form of NOTCH1), downregulated HES1 and MYC, but slightly increased NOTCH2 levels, whereas NOTCH2 knockdown slightly upregulated the levels of cleaved NOTCH1 fragment, HES1, MYC, and NOTCH1. In KOPT-K1 cells, NOTCH1 knockdown decreased the expression of cleaved NOTCH1 fragment and HES1, while slightly increasing that of NOTCH2, whereas NOTCH2 knockdown did not affect the expression of other proteins. The knockdown of NOTCH1 and 2 did not affect the level of mTOR, Hedgehog, and Wnt signaling-related proteins in both cell lines.

Conclusion
In this study, we confirmed our previous findings that GSI-XXI treatment suppressed the growth of KOPT-K1 cells while promoting that of Jurkat cells using NOTCH1- and NOTCH2-specific siRNA. It is well known that NOTCH activation is oncogenic and promotes the growth of T-ALL cells. However, in Jurkat cells NOTCH obviously exerted the opposite effect. Interestingly, the knockdown of NOTCH2, similar to that of NOTCH1, affected the growth of the two cell lines, although they do not have NOTCH2-activating mutations. Furthermore, in Jurkat cells, we observed an interesting phenomenon that NOTCH1 knockdown upregulated NOTCH2 and vice versa, which might be related to the cell proliferation due to NOTCH suppression although this mechanism can not fully answer the question in the aim of this study. The underlying molecular mechanisms and the biological significance of the effect need to be further elucidated. Our results contribute to understanding of the role of NOTCH signaling in T-ALL cells and should promote the development of novel NOTCH-targeting therapies against T-ALL.
Session topic: 1. Acute lymphoblastic leukemia – Biology & Translational Research
Keyword(s): Notch, SiRNA, T cell acute lymphoblastic leukemia


