
Contributions
Abstract: PB1601
Type: Publication Only
Background
Relapse remains the major cause of treatment failure in adults with B-cell acute lymphoblastic leukemia (ALL) achieving complete remission after induction therapy. Identifying new biomarkers in B-cell ALL and studying their clinical significance and biological function will be helpful for risk-stratification, treatment decision and targeted therapy. RASD1 (ras related dexamethasone induced 1) maps to chromosome sub-band 17p11.2, which encodes a member of the Ras superfamily of small GTPases. The role of RASD1 in cancer remains controversial. In our previously conducted bio-informatics analyses of transcriptomic data to identify mRNA transcripts aberrantly-expressed in B-cell ALL, RASD1 was identified as one of most differentially expressed genes which were up-regulated in B-cell ALL. However, the expression and biological function of RASD1 remain unknown in B-cell ALL.
Aims
To investigate the expression level of human RASD1 messenger RNA in B-cell ALL by real-time fluorescent quantitative reverse transcription-polymerase chain reaction assay.
Methods
A real-time quantitative RT-PCR based on TaqMan fluorescence methodology was used to examine RASD1 expression in 11 malignant hematological disorders cell lines and bone marrow samples from 158 adults with B-cell ALL and 40 healthy donors.
Results
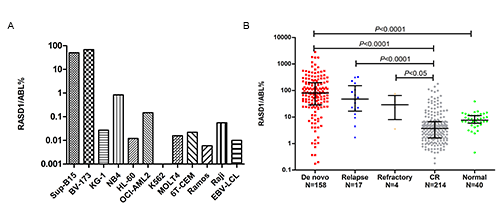
Results showed that RASD1 gene expression was higher in cell lines from B-cell ALL (Sup-B15, BV-173), but nearly undetectable in cell lines derived from AML(KG-1, NB4, HL60, OCI-AML2), CML(K562), T-ALL(MOLT4, 6T-CEM), lympoma(Ramos, Raji) and Epstein-Barr virus-transformed lymphoblastoid cell lines (EBV-LCL)(Fig.1A). The relative levels of RASD1 gene expression in marrow from the 158 newly diagnosed B-cell ALL (median 80.12%; range 0.17-2822.33%) were significantly higher than those of marrow from the 40 healthy donors(median 7.59%; range 0.46-38.66%, p<0.0001, Fig.1B). The median level of RASD1 in 158 newly diagnosed patients was 80.12%, while the median level in 214 treated patients who achieved complete remission decreased to 3.71%. However, in 17 relapsed patients and 4 refractory patients, the median level was 47.29% and 29.01%, respectively(Fig.1B). In newly diagnosed B-cell ALL, 4 patients with MLL-AF4 translocation showed lower RASD1 expression compared with 68 patients without this translocation (p=0.005). RASD1 expression levels were not significantly associated with initial white blood cell counts, hemoglobin, and platelet counts in the peripheral blood, blast in the bone marrow, age, sex, prognosis grouping, immunophenotype, BCR-ABL fusion gene and IKZF1 deletion (p>0.05).
Fig.1 Levels of RASD1 expression in (A) leukemic cell lines; (B) de novo, relapsed, refractory and complete remission (CR) B-cell ALL and normal bone marrow.
Conclusion
These findings suggest that RASD1 was widely over-expressed in adults with B-cell ALL and that abnormal expression of RASD1 in leukemia may be involved in the pathomechanism of B-cell ALL.
Session topic: 1. Acute lymphoblastic leukemia – Biology & Translational Research
Keyword(s): Acute lymphoblastic leukemia, Molecular markers, Overexpression, RQ-PCR
Abstract: PB1601
Type: Publication Only
Background
Relapse remains the major cause of treatment failure in adults with B-cell acute lymphoblastic leukemia (ALL) achieving complete remission after induction therapy. Identifying new biomarkers in B-cell ALL and studying their clinical significance and biological function will be helpful for risk-stratification, treatment decision and targeted therapy. RASD1 (ras related dexamethasone induced 1) maps to chromosome sub-band 17p11.2, which encodes a member of the Ras superfamily of small GTPases. The role of RASD1 in cancer remains controversial. In our previously conducted bio-informatics analyses of transcriptomic data to identify mRNA transcripts aberrantly-expressed in B-cell ALL, RASD1 was identified as one of most differentially expressed genes which were up-regulated in B-cell ALL. However, the expression and biological function of RASD1 remain unknown in B-cell ALL.
Aims
To investigate the expression level of human RASD1 messenger RNA in B-cell ALL by real-time fluorescent quantitative reverse transcription-polymerase chain reaction assay.
Methods
A real-time quantitative RT-PCR based on TaqMan fluorescence methodology was used to examine RASD1 expression in 11 malignant hematological disorders cell lines and bone marrow samples from 158 adults with B-cell ALL and 40 healthy donors.
Results
Results showed that RASD1 gene expression was higher in cell lines from B-cell ALL (Sup-B15, BV-173), but nearly undetectable in cell lines derived from AML(KG-1, NB4, HL60, OCI-AML2), CML(K562), T-ALL(MOLT4, 6T-CEM), lympoma(Ramos, Raji) and Epstein-Barr virus-transformed lymphoblastoid cell lines (EBV-LCL)(Fig.1A). The relative levels of RASD1 gene expression in marrow from the 158 newly diagnosed B-cell ALL (median 80.12%; range 0.17-2822.33%) were significantly higher than those of marrow from the 40 healthy donors(median 7.59%; range 0.46-38.66%, p<0.0001, Fig.1B). The median level of RASD1 in 158 newly diagnosed patients was 80.12%, while the median level in 214 treated patients who achieved complete remission decreased to 3.71%. However, in 17 relapsed patients and 4 refractory patients, the median level was 47.29% and 29.01%, respectively(Fig.1B). In newly diagnosed B-cell ALL, 4 patients with MLL-AF4 translocation showed lower RASD1 expression compared with 68 patients without this translocation (p=0.005). RASD1 expression levels were not significantly associated with initial white blood cell counts, hemoglobin, and platelet counts in the peripheral blood, blast in the bone marrow, age, sex, prognosis grouping, immunophenotype, BCR-ABL fusion gene and IKZF1 deletion (p>0.05).
Fig.1 Levels of RASD1 expression in (A) leukemic cell lines; (B) de novo, relapsed, refractory and complete remission (CR) B-cell ALL and normal bone marrow.

Conclusion
These findings suggest that RASD1 was widely over-expressed in adults with B-cell ALL and that abnormal expression of RASD1 in leukemia may be involved in the pathomechanism of B-cell ALL.
Session topic: 1. Acute lymphoblastic leukemia – Biology & Translational Research
Keyword(s): Acute lymphoblastic leukemia, Molecular markers, Overexpression, RQ-PCR


