
Contributions
Abstract: PB2437
Type: Publication Only
Background
Relapse after allogeneic stem cell transplantation (post-allo-SCT) is the main cause of treatment failure. Currently, the incidence of relapse in patients with acute myeloid leukemia (AML) is between 30-40%. The mean overall survival is 3 to 4 months and the overall survival at two years is less than 20%.
Aims
The aim of this study was to define the benefits of Azacitidine (AZA) in relapsed patients post-allo-SCT, looking for an association between the post-relapse overall survival and the response rate to AZA. We also examined FBC values (increase in hemoglobin, neutrophil or platelet counts), cytogenetic abnormalities present at relapse, and variations in chimerism.
Methods
This is a retrospective single-center study, which analyzed 14 patients who received AZA from January 2013 to January 2018 at a dose of 75mg / m². The cytogenetic risk groups were established according to the European Leukemia Net classification. We used the Kaplan-Meier statistical analysis to estimate the postrelapse overall survival, and we analyzed the impact with the Log-Rank and Breslow tests.
Results
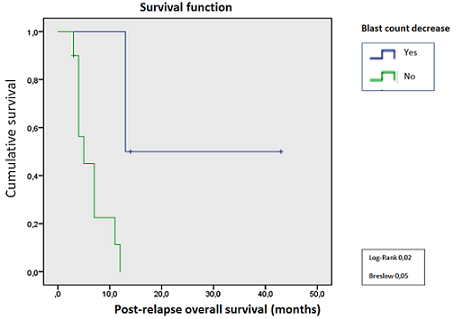
The patient’s characteristics are shown in Table 1. The mean age was 54 (± 12) years. The mean time to relapse post-transplant was 7.4 (± 4.9) months. The mean treatment time with AZA was 4 (± 2.5) months, with an average of 4 (± 2) cycles, and a mean cycle length of 6 (± 1) days per cycle. A decrease in the blast percentage occurred in 4 patients, one of them reached a complete remission. We found a statistically significant association (p <0.05) between the treatment response measured by blast count decrease, and the overall survival (graph 1). The average duration of the response was 12 months. One of these 4 patients presented a hematological response with an increase in the hemoglobin level, another showed an increase in platelet count, and a third presented an increase in both hemoglobin and in the neutrophil count. Two patients presented a cytogenetic response with post-AZA negativization of high-risk genetic markers: Del (5q) and del (7q) in one patient, and inv (3) (q21q26) in the other. Changes in chimerism occurred in four patients, two of them achieved a complete chimerism. The median overall survival was 7 (3-14) months. However, even patients who presented a hematological response, a decrease in the percentage of blasts, normalization of cytogenetic abnormalities or who achieved a complete chimerism, lost those responses with the progression of the underlying disease. Table1.
Characteristics |
| N | % | Results postAZA |
| N | % |
Sex | Female Male | 9 5 | 64 36 | Blast | Descent without RC Descent with RC Progressión | 3 1 10 | 21 7 72 |
Cytogenetics in AML | Good Intermediate Adverse | 0 1 13 | 0 7 93 | Cytogenetic response | Yes No | 2 12 | 14 86 |
Conditioning | Haploidentical MAC RIC | 10 2 2 | 72 14 14 | Change chimerism | Yes No | 4 10 | 29 71 |
AZA indication | Frank relapse EMR+ Genetic marker+ | 12 1 1 | 86 2 2 |
| |||
Early relapse (≤6 months) | Yes No | 8 6 | 57 43 |
| |||
Conclusion
AZA is a therapeutic option in post allo-SCT relapsed patients. In our study, the median overall survival was 7 (3-14) months, which is longer than expected in this group of patients. In addition, AZA can modify characteristics of the disease, such as genetic markers of poor prognosis and chimerism. Althought many patients present an initial response, this is then lost with disease progression. The development of new therapeutic strategies is needed in this cohort of patients, to offer a better prognosis in post allo-SCT relapse.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Acute Myeloid Leukemia, Allogeneic stem cell transplant, Azacitidine, Relapse
Abstract: PB2437
Type: Publication Only
Background
Relapse after allogeneic stem cell transplantation (post-allo-SCT) is the main cause of treatment failure. Currently, the incidence of relapse in patients with acute myeloid leukemia (AML) is between 30-40%. The mean overall survival is 3 to 4 months and the overall survival at two years is less than 20%.
Aims
The aim of this study was to define the benefits of Azacitidine (AZA) in relapsed patients post-allo-SCT, looking for an association between the post-relapse overall survival and the response rate to AZA. We also examined FBC values (increase in hemoglobin, neutrophil or platelet counts), cytogenetic abnormalities present at relapse, and variations in chimerism.
Methods
This is a retrospective single-center study, which analyzed 14 patients who received AZA from January 2013 to January 2018 at a dose of 75mg / m². The cytogenetic risk groups were established according to the European Leukemia Net classification. We used the Kaplan-Meier statistical analysis to estimate the postrelapse overall survival, and we analyzed the impact with the Log-Rank and Breslow tests.
Results
The patient’s characteristics are shown in Table 1. The mean age was 54 (± 12) years. The mean time to relapse post-transplant was 7.4 (± 4.9) months. The mean treatment time with AZA was 4 (± 2.5) months, with an average of 4 (± 2) cycles, and a mean cycle length of 6 (± 1) days per cycle. A decrease in the blast percentage occurred in 4 patients, one of them reached a complete remission. We found a statistically significant association (p <0.05) between the treatment response measured by blast count decrease, and the overall survival (graph 1). The average duration of the response was 12 months. One of these 4 patients presented a hematological response with an increase in the hemoglobin level, another showed an increase in platelet count, and a third presented an increase in both hemoglobin and in the neutrophil count. Two patients presented a cytogenetic response with post-AZA negativization of high-risk genetic markers: Del (5q) and del (7q) in one patient, and inv (3) (q21q26) in the other. Changes in chimerism occurred in four patients, two of them achieved a complete chimerism. The median overall survival was 7 (3-14) months. However, even patients who presented a hematological response, a decrease in the percentage of blasts, normalization of cytogenetic abnormalities or who achieved a complete chimerism, lost those responses with the progression of the underlying disease. Table1.
Characteristics |
| N | % | Results postAZA |
| N | % |
Sex | Female Male | 9 5 | 64 36 | Blast | Descent without RC Descent with RC Progressión | 3 1 10 | 21 7 72 |
Cytogenetics in AML | Good Intermediate Adverse | 0 1 13 | 0 7 93 | Cytogenetic response | Yes No | 2 12 | 14 86 |
Conditioning | Haploidentical MAC RIC | 10 2 2 | 72 14 14 | Change chimerism | Yes No | 4 10 | 29 71 |
AZA indication | Frank relapse EMR+ Genetic marker+ | 12 1 1 | 86 2 2 |
| |||
Early relapse (≤6 months) | Yes No | 8 6 | 57 43 |
| |||

Conclusion
AZA is a therapeutic option in post allo-SCT relapsed patients. In our study, the median overall survival was 7 (3-14) months, which is longer than expected in this group of patients. In addition, AZA can modify characteristics of the disease, such as genetic markers of poor prognosis and chimerism. Althought many patients present an initial response, this is then lost with disease progression. The development of new therapeutic strategies is needed in this cohort of patients, to offer a better prognosis in post allo-SCT relapse.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Acute Myeloid Leukemia, Allogeneic stem cell transplant, Azacitidine, Relapse


