
Contributions
Abstract: PB2252
Type: Publication Only
Background
In NDMM, following response to primary therapy, maintenance therapy prolongs progression-free survival (PFS) and overall survival (OS) (McCarthy et al, J Clin Oncol 2017; Ludwig et al, Blood 2012). However, although multiple drugs have been investigated, maintenance is not yet an established treatment option worldwide; lenalidomide is the only approved agent, and only as post-autologous stem cell transplant (ASCT) maintenance.
Aims
We reviewed published/ongoing phase 3 trials of current and emerging maintenance treatment options to evaluate the treatment landscape and identify unmet medical needs and how these needs might be addressed.
Methods
A focused literature review and a search of clinical trial registries were conducted to identify publications and ongoing phase 3 studies of maintenance treatment approaches in NDMM.
Results
Maintenance with lenalidomide, bortezomib, or thalidomide has been well-studied and offers differential benefit, leaving unmet needs in some populations. Lenalidomide maintenance post-ASCT prolongs PFS and OS versus placebo/observation (McCarthy et al, J Clin Oncol 2017) but increases risk of second primary malignancies and has a less pronounced benefit in some subgroups, including patients with high-risk cytogenetics and those with high ISS stage. Lenalidomide has shown improved PFS, but not OS, in the non-ASCT setting (Palumbo et al, N Engl J Med 2012). Thalidomide maintenance demonstrates a significant risk reduction for PFS, but not in patients with high-risk cytogenetics (Ludwig et al, Blood 2012), and with notable toxicity. Although both lenalidomide and thalidomide have shown OS benefits, these benefits are not consistent across all individual studies. Bortezomib has activity post-ASCT, including in patients with high-risk cytogenetics (Sonneveld et al, J Clin Oncol 2012), and in the non-ASCT setting (Palumbo et al, J Clin Oncol 2010; Mateos et al, Lancet Oncol 2010), but has not been studied in a placebo-controlled setting. Long-term use of bortezomib and thalidomide may be limited by short- and long-term toxicity, such as peripheral neuropathy, as well as by the treatment burden associated with repeated parenteral administration.
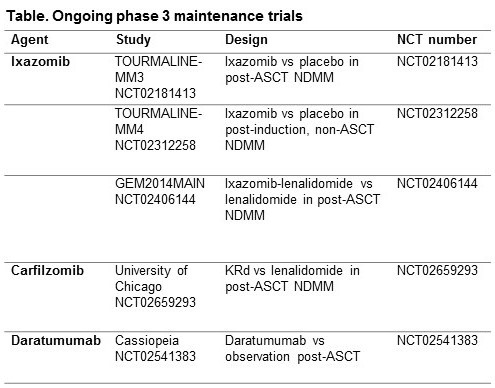
Although these agents are being used for maintenance, several unmet medical needs still remain, including the need for a therapy that can be dosed for an extended time without cumulative or late-onset toxicity or the emergence of resistant clones at relapse. Further, there is a need for a maintenance therapy offering extended benefit in all patients, including subgroups with high-risk disease (advanced stage, high tumor burden, high-risk cytogenetics, comorbidities). Ideally, maintenance should deepen patient responses instead of just sustaining an existing response. There are several ongoing phase 3 studies of ixazomib, carfilzomib, and daratumumab as maintenance (Table) that may address some of the unmet needs. Long-term treatment with regimens containing these agents results in deepening responses and improved outcomes, including in multiple high-risk patient subgroups. Oral ixazomib potentially offers a convenient and feasible approach to long-term proteasome inhibitor therapy, with a manageable toxicity profile.
Conclusion
Current maintenance therapies for NDMM have resulted in improved long-term outcomes but are associated with some limitations. Emerging therapies may offer a feasible approach due to manageable long-term toxicity profiles and convenience, especially oral agents.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Maintenance, Myeloma, Phase III
Abstract: PB2252
Type: Publication Only
Background
In NDMM, following response to primary therapy, maintenance therapy prolongs progression-free survival (PFS) and overall survival (OS) (McCarthy et al, J Clin Oncol 2017; Ludwig et al, Blood 2012). However, although multiple drugs have been investigated, maintenance is not yet an established treatment option worldwide; lenalidomide is the only approved agent, and only as post-autologous stem cell transplant (ASCT) maintenance.
Aims
We reviewed published/ongoing phase 3 trials of current and emerging maintenance treatment options to evaluate the treatment landscape and identify unmet medical needs and how these needs might be addressed.
Methods
A focused literature review and a search of clinical trial registries were conducted to identify publications and ongoing phase 3 studies of maintenance treatment approaches in NDMM.
Results
Maintenance with lenalidomide, bortezomib, or thalidomide has been well-studied and offers differential benefit, leaving unmet needs in some populations. Lenalidomide maintenance post-ASCT prolongs PFS and OS versus placebo/observation (McCarthy et al, J Clin Oncol 2017) but increases risk of second primary malignancies and has a less pronounced benefit in some subgroups, including patients with high-risk cytogenetics and those with high ISS stage. Lenalidomide has shown improved PFS, but not OS, in the non-ASCT setting (Palumbo et al, N Engl J Med 2012). Thalidomide maintenance demonstrates a significant risk reduction for PFS, but not in patients with high-risk cytogenetics (Ludwig et al, Blood 2012), and with notable toxicity. Although both lenalidomide and thalidomide have shown OS benefits, these benefits are not consistent across all individual studies. Bortezomib has activity post-ASCT, including in patients with high-risk cytogenetics (Sonneveld et al, J Clin Oncol 2012), and in the non-ASCT setting (Palumbo et al, J Clin Oncol 2010; Mateos et al, Lancet Oncol 2010), but has not been studied in a placebo-controlled setting. Long-term use of bortezomib and thalidomide may be limited by short- and long-term toxicity, such as peripheral neuropathy, as well as by the treatment burden associated with repeated parenteral administration.
Although these agents are being used for maintenance, several unmet medical needs still remain, including the need for a therapy that can be dosed for an extended time without cumulative or late-onset toxicity or the emergence of resistant clones at relapse. Further, there is a need for a maintenance therapy offering extended benefit in all patients, including subgroups with high-risk disease (advanced stage, high tumor burden, high-risk cytogenetics, comorbidities). Ideally, maintenance should deepen patient responses instead of just sustaining an existing response. There are several ongoing phase 3 studies of ixazomib, carfilzomib, and daratumumab as maintenance (Table) that may address some of the unmet needs. Long-term treatment with regimens containing these agents results in deepening responses and improved outcomes, including in multiple high-risk patient subgroups. Oral ixazomib potentially offers a convenient and feasible approach to long-term proteasome inhibitor therapy, with a manageable toxicity profile.

Conclusion
Current maintenance therapies for NDMM have resulted in improved long-term outcomes but are associated with some limitations. Emerging therapies may offer a feasible approach due to manageable long-term toxicity profiles and convenience, especially oral agents.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Maintenance, Myeloma, Phase III


