
Contributions
Abstract: PB1876
Type: Publication Only
Background
The current first-line treatment for fit chronic lymphocytic leukemia (CLL) patients remains fludarabine-based therapy. Elderly patients with numerous comorbidities, poorly tolerate such regimen and pose a huge challenge.
Aims
In our analysis, we investigated the efficacy and safety obinutuzumab-chlorambucil combination in elderly and unfit patients.
Methods
We include in our analysis 86 treatment-naïve CLL patients (median age 74 years, range 51 – 86 years) with significant burden of coexisting comorbidities. All patients presented the Cumulative Illness Rating Scale (CIRS) score greater than 6 and/or creatinine clearance (CrCl) of 30–69 ml/min. Most patients (94,19%) had four or more coexisting comorbidities, with cardiovascular, endocrine or metabolic, respiratory and genitourinary disorders being the most frequent. Obinutuzumab was infused intravenously at 1000 mg on days 1, 8 and 15 of cycle 1 and on day 1 of cycles 2–6 (28-day cycles) with first infusion split over 2 days for patients’ safety. Chlorambucil was administered orally at a dose of 0.5 mg per kilogram of body weight on days 1 and 15 of each cycle.
Results
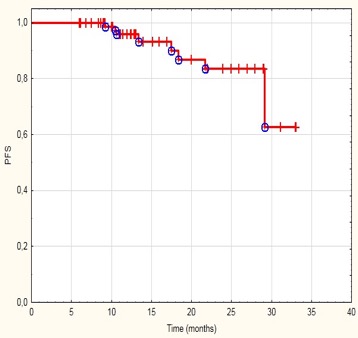
Overall response rate (ORR) at 2 months after treatment completion was 95.35% including complete remission (CR) in 37 patients (45.12%) and partial remission (PR) in 45 patients (54.88%). Stable disease was noted in 4 patients (4.65%) and progressive disease (PD) was not observed after the end of therapy. The median progression free survival (PFS) was 13,05 months. The relapses occurred in 6 patients (7%) with 3 patients (3.5%) completing treatment with CR and 3 (3.5%) with PR. The median number of treatment cycles was 6. The most frequent adverse events (AE) were infusion-related reactions (IRR) and neutropenia. Grade 3 IRR occurred in 2.3 % of patients, grade 2 in 12.8% (in 81.8% of patients only during the first infusion of monoclonal antibody). Grade 3 neutropenia was reported in 11.6% of patients, grade 2 in 22.1% with no incidence of febrile neutropenia. There were no AE of grade 4 or 5.
Conclusion
Our data confirm that obinutuzumab-chlorambucil is an effective and well-tolerated regimen in untreated CLL patients with comorbidities.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Chronic Lymphocytic Leukemia, Comorbidities, Obinutuzumab
Abstract: PB1876
Type: Publication Only
Background
The current first-line treatment for fit chronic lymphocytic leukemia (CLL) patients remains fludarabine-based therapy. Elderly patients with numerous comorbidities, poorly tolerate such regimen and pose a huge challenge.
Aims
In our analysis, we investigated the efficacy and safety obinutuzumab-chlorambucil combination in elderly and unfit patients.
Methods
We include in our analysis 86 treatment-naïve CLL patients (median age 74 years, range 51 – 86 years) with significant burden of coexisting comorbidities. All patients presented the Cumulative Illness Rating Scale (CIRS) score greater than 6 and/or creatinine clearance (CrCl) of 30–69 ml/min. Most patients (94,19%) had four or more coexisting comorbidities, with cardiovascular, endocrine or metabolic, respiratory and genitourinary disorders being the most frequent. Obinutuzumab was infused intravenously at 1000 mg on days 1, 8 and 15 of cycle 1 and on day 1 of cycles 2–6 (28-day cycles) with first infusion split over 2 days for patients’ safety. Chlorambucil was administered orally at a dose of 0.5 mg per kilogram of body weight on days 1 and 15 of each cycle.
Results
Overall response rate (ORR) at 2 months after treatment completion was 95.35% including complete remission (CR) in 37 patients (45.12%) and partial remission (PR) in 45 patients (54.88%). Stable disease was noted in 4 patients (4.65%) and progressive disease (PD) was not observed after the end of therapy. The median progression free survival (PFS) was 13,05 months. The relapses occurred in 6 patients (7%) with 3 patients (3.5%) completing treatment with CR and 3 (3.5%) with PR. The median number of treatment cycles was 6. The most frequent adverse events (AE) were infusion-related reactions (IRR) and neutropenia. Grade 3 IRR occurred in 2.3 % of patients, grade 2 in 12.8% (in 81.8% of patients only during the first infusion of monoclonal antibody). Grade 3 neutropenia was reported in 11.6% of patients, grade 2 in 22.1% with no incidence of febrile neutropenia. There were no AE of grade 4 or 5.

Conclusion
Our data confirm that obinutuzumab-chlorambucil is an effective and well-tolerated regimen in untreated CLL patients with comorbidities.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Chronic Lymphocytic Leukemia, Comorbidities, Obinutuzumab


