
Contributions
Abstract: PB1867
Type: Publication Only
Background
Approximately 1800 individuals live with chronic lymphocytic leukemia (CLL) in Finland, with 350 diagnosed annually. Real-world treatment outcomes for this patient (pt) group have not been assessed previously in Finland and in the absence of direct head-to-head trials, robust historical control data have become important to ascertain the efficacy of novel therapies prior to introduction. The Finnish Hematology Registry (FHR) was created to allow for the collection of real-world practice outcomes in CLL and other hematological malignancies, with Finland’s centralized nationwide healthcare system enabling reliable pt identification and comprehensive follow-up.
Aims
The aim of this registry study is to describe treatment and survival outcomes for CLL pts from routine practice settings, with special emphasis on second line treatment.
Methods
A non-interventional, retrospective study design was used to collect routine clinical practice data from the FHR. Eligible pts aged ≥18 years diagnosed with CLL and receiving 1 or more treatment lines during 2005-2015 were identified. As the registry had limited CLL pt follow-up data at study start, clinical investigators accessed medical records to retrieve and validate data. We report on 124 pts who met inclusion criteria from the Helsinki University Hospital, Helsinki, Finland, a hospital region accounting for 30% of the total national CLL incidence. Second line pts (n=64) were further stratified into those starting their treatment in 2006-2010 and in 2011-2015 time period, and treatment outcomes are described per time period and select treatments. For this initial analysis of 2nd line outcomes, crude overall survival (OS) and time-to-next-treatment (TTNT) were calculated per treatment line using Kaplan–Meier methods, with log-rank to estimate p-values.
Results
Median time to second line treatment was 31 months (m). During the follow-up, subtle changes in overall treatment practice were observed with fludarabine-cyclophosphamide-rituximab (FCR) and bendamustine-rituximab (BR) becoming the mainstay over fludarabine-cyclophosphamide (FC) after 2008. Across second line treatments, B/BR (36%), FCR (17%), chlorambucil-based therapies (20%) and (FC) (8%) were the most frequently used treatments. Novel agents ibrutinib, idelalisib+rituximab and obinutuzumab were not used prior to 2015.
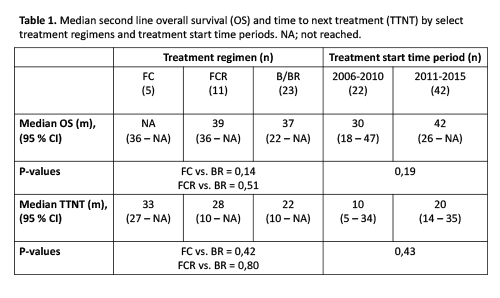
Median OS and TTNT from the start of any given 2nd line treatment were 37 m and 19 m, respectively. Pts receiving B/BR had shorter, though non-significant, median OS (37 m) compared to FCR (39 m) (p=0,51) (Table 1). TTNT was longer for pts receiving FC (33 m, p=0,42) and FCR (28 m, p=0,80) compared to B/BR (22 m). Despite apparent changes in treatment practice and increased use of chemoimmunotherapy over the years, improvements in 2nd line median OS (30 vs. 42 m, p=0,19) and TTNT (10 vs. 20, p=0,43) for the period 2006-2010 vs. 2011-2015 did not reach statistical significance.
Conclusion
We describe real-world CLL treatment trends and outcomes from a decade of practice in the Helsinki hospital region focusing on 2nd line regimens. These results are consistent with reports from Sweden (Asklid et al. 2016). Changes in treatment practice were observed between different time periods, however, results do not show significant improvements for OS and TTNT during this decade of follow-up. In the Nordics, real world evidence has become essential when introducing novel targeted therapies into clinical practice settings, with comprehensive disease registries expanding to allow for more detailed follow-up.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): chemotherapy, Chronic Lymphocytic Leukemia, epidemiology, Outcome
Abstract: PB1867
Type: Publication Only
Background
Approximately 1800 individuals live with chronic lymphocytic leukemia (CLL) in Finland, with 350 diagnosed annually. Real-world treatment outcomes for this patient (pt) group have not been assessed previously in Finland and in the absence of direct head-to-head trials, robust historical control data have become important to ascertain the efficacy of novel therapies prior to introduction. The Finnish Hematology Registry (FHR) was created to allow for the collection of real-world practice outcomes in CLL and other hematological malignancies, with Finland’s centralized nationwide healthcare system enabling reliable pt identification and comprehensive follow-up.
Aims
The aim of this registry study is to describe treatment and survival outcomes for CLL pts from routine practice settings, with special emphasis on second line treatment.
Methods
A non-interventional, retrospective study design was used to collect routine clinical practice data from the FHR. Eligible pts aged ≥18 years diagnosed with CLL and receiving 1 or more treatment lines during 2005-2015 were identified. As the registry had limited CLL pt follow-up data at study start, clinical investigators accessed medical records to retrieve and validate data. We report on 124 pts who met inclusion criteria from the Helsinki University Hospital, Helsinki, Finland, a hospital region accounting for 30% of the total national CLL incidence. Second line pts (n=64) were further stratified into those starting their treatment in 2006-2010 and in 2011-2015 time period, and treatment outcomes are described per time period and select treatments. For this initial analysis of 2nd line outcomes, crude overall survival (OS) and time-to-next-treatment (TTNT) were calculated per treatment line using Kaplan–Meier methods, with log-rank to estimate p-values.
Results
Median time to second line treatment was 31 months (m). During the follow-up, subtle changes in overall treatment practice were observed with fludarabine-cyclophosphamide-rituximab (FCR) and bendamustine-rituximab (BR) becoming the mainstay over fludarabine-cyclophosphamide (FC) after 2008. Across second line treatments, B/BR (36%), FCR (17%), chlorambucil-based therapies (20%) and (FC) (8%) were the most frequently used treatments. Novel agents ibrutinib, idelalisib+rituximab and obinutuzumab were not used prior to 2015.
Median OS and TTNT from the start of any given 2nd line treatment were 37 m and 19 m, respectively. Pts receiving B/BR had shorter, though non-significant, median OS (37 m) compared to FCR (39 m) (p=0,51) (Table 1). TTNT was longer for pts receiving FC (33 m, p=0,42) and FCR (28 m, p=0,80) compared to B/BR (22 m). Despite apparent changes in treatment practice and increased use of chemoimmunotherapy over the years, improvements in 2nd line median OS (30 vs. 42 m, p=0,19) and TTNT (10 vs. 20, p=0,43) for the period 2006-2010 vs. 2011-2015 did not reach statistical significance.

Conclusion
We describe real-world CLL treatment trends and outcomes from a decade of practice in the Helsinki hospital region focusing on 2nd line regimens. These results are consistent with reports from Sweden (Asklid et al. 2016). Changes in treatment practice were observed between different time periods, however, results do not show significant improvements for OS and TTNT during this decade of follow-up. In the Nordics, real world evidence has become essential when introducing novel targeted therapies into clinical practice settings, with comprehensive disease registries expanding to allow for more detailed follow-up.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): chemotherapy, Chronic Lymphocytic Leukemia, epidemiology, Outcome


