
Contributions
Abstract: PB1730
Type: Publication Only
Background
There is paucity of published data regarding the treatment strategy of patients infected at baseline prior to initiation of induction chemotherapy.
Aims
To determine the impact of infection at baseline(prior to the initiation of chemotherapy) in acute leukemia patients undergoing induction chemotherapy with respect to mortality and CR rates
Methods
Ambispectively (from 1-7-2015 to 31-12-2017) we included newly diagnosed patients with acute leukemia undergoing induction chemotherapy.
Results
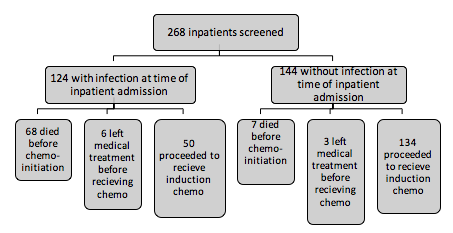
A total of 268 admitted in-patients of acute leukemia were screened in the Dept of Hematology at All india Institute of Medical sciences(AIIMS), New Delhi, India . Out of these 124 admitted patients were found to have infections at baseline prior to initiation of induction chemotherapy. A large proportion of admitted infected acute leukemia patients died before the initiation of induction chemotherapy (54.83%) .The median duration of illness prior to admission in the group of infected patients who later proceeded on to receive induction chemotherapy was longer in comparison to patients without infection and this difference was statistically significant (12 weeks versus 6 weeks, p< 0.00001 ). Ninety-six percentage of the patients with baseline infection prior to initiation of induction chemotherapy were treated with IV antibiotics and antifungals each. The mean duration of therapeutic antibiotic therapy and antifungal therapy prior to induction chemotherapy was 16.08 days and 13.6 days respectively. Prior to the initiation of induction chemotherapy it was determined that clinically 78% of patients had improvement in the status of their infection, twelve percentage had complete resolution of the documented baseline infection.In the patients with baseline infection, after initiation of induction chemotherapy, the majority (82%) developed either new infections and/or worsening of pre-existing infections in comparison to the group without infection at baseline (53.73%) , and this difference was statistically significant(p=0.0005).Lesser proportion of the uninfected patients at baseline developed febrile neutropenia (53.73%) in comparison to the group with baseline infections (80%) upon induction chemotherapy initiation, and this difference was statistically significant(p=0.0012). After the initiation of chemotherapy the occurrence of septic shock in the group of patients with infection at baseline (42%) was more frequent than in the group without infection at baseline (22.388%) and this difference was statistically significant (p=0.0084). The difference in the induction mortality rates in both groups was not significant statistically (32 % in baseline infected group versus 23.13% in the uninfected group, p=0.1968), even on subset analysis comparing AML/ALL of either groups individually; AML (40% versus 29.16% , p= 0.3262) /ALL(22.2% versus 18.18%, p=0.7019) .The CR rates in the baseline uninfected group versus in the baseline infected group was 78.78% versus 61.11% respectively in ALL and was 39.58% versus 36.66% in AML and neither of the difference was statistically significant.
Conclusion
Patients with infection at baseline demonstrating clinical improvement upon treatment with antibiotics/antifungals can be considered for induction chemotherapy. There is need for studies using newer therapeutic agents with potentially lower disruption of muco-cutaneous barriers , lower myelotoxicity and potential infliction of lesser physiological stress as a bridge to subsequent full dose induction chemotherapy in these patients infected at baseline even prior to the initiation of chemotherapy.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): ALL, AML, Induction chemotherapy, Infection
Abstract: PB1730
Type: Publication Only
Background
There is paucity of published data regarding the treatment strategy of patients infected at baseline prior to initiation of induction chemotherapy.
Aims
To determine the impact of infection at baseline(prior to the initiation of chemotherapy) in acute leukemia patients undergoing induction chemotherapy with respect to mortality and CR rates
Methods
Ambispectively (from 1-7-2015 to 31-12-2017) we included newly diagnosed patients with acute leukemia undergoing induction chemotherapy.
Results
A total of 268 admitted in-patients of acute leukemia were screened in the Dept of Hematology at All india Institute of Medical sciences(AIIMS), New Delhi, India . Out of these 124 admitted patients were found to have infections at baseline prior to initiation of induction chemotherapy. A large proportion of admitted infected acute leukemia patients died before the initiation of induction chemotherapy (54.83%) .The median duration of illness prior to admission in the group of infected patients who later proceeded on to receive induction chemotherapy was longer in comparison to patients without infection and this difference was statistically significant (12 weeks versus 6 weeks, p< 0.00001 ). Ninety-six percentage of the patients with baseline infection prior to initiation of induction chemotherapy were treated with IV antibiotics and antifungals each. The mean duration of therapeutic antibiotic therapy and antifungal therapy prior to induction chemotherapy was 16.08 days and 13.6 days respectively. Prior to the initiation of induction chemotherapy it was determined that clinically 78% of patients had improvement in the status of their infection, twelve percentage had complete resolution of the documented baseline infection.In the patients with baseline infection, after initiation of induction chemotherapy, the majority (82%) developed either new infections and/or worsening of pre-existing infections in comparison to the group without infection at baseline (53.73%) , and this difference was statistically significant(p=0.0005).Lesser proportion of the uninfected patients at baseline developed febrile neutropenia (53.73%) in comparison to the group with baseline infections (80%) upon induction chemotherapy initiation, and this difference was statistically significant(p=0.0012). After the initiation of chemotherapy the occurrence of septic shock in the group of patients with infection at baseline (42%) was more frequent than in the group without infection at baseline (22.388%) and this difference was statistically significant (p=0.0084). The difference in the induction mortality rates in both groups was not significant statistically (32 % in baseline infected group versus 23.13% in the uninfected group, p=0.1968), even on subset analysis comparing AML/ALL of either groups individually; AML (40% versus 29.16% , p= 0.3262) /ALL(22.2% versus 18.18%, p=0.7019) .The CR rates in the baseline uninfected group versus in the baseline infected group was 78.78% versus 61.11% respectively in ALL and was 39.58% versus 36.66% in AML and neither of the difference was statistically significant.

Conclusion
Patients with infection at baseline demonstrating clinical improvement upon treatment with antibiotics/antifungals can be considered for induction chemotherapy. There is need for studies using newer therapeutic agents with potentially lower disruption of muco-cutaneous barriers , lower myelotoxicity and potential infliction of lesser physiological stress as a bridge to subsequent full dose induction chemotherapy in these patients infected at baseline even prior to the initiation of chemotherapy.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): ALL, AML, Induction chemotherapy, Infection


